CARBOCATIONS: STRUCTURE AND REACTIVITY
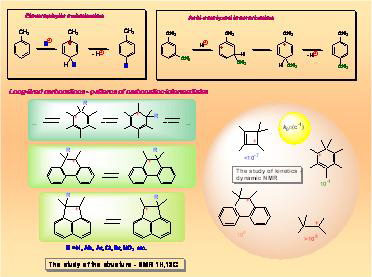
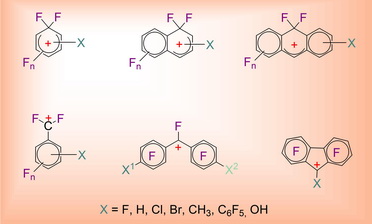
In a series of studies conducted under the leadership of Academician V.A. Koptyug with the use of modern physical methods (NMR, spectroscopy, X-ray diffraction method, etc.) there have been obtained unique data on electron and spatial structure of the main types of carbocations modeling reactive intermediates of such practically important organic reactions as electrophilic substitution, isomerization, condensation of organic compounds and cationoid molecular rearrangements.
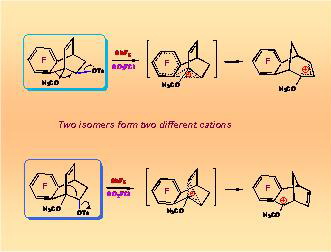
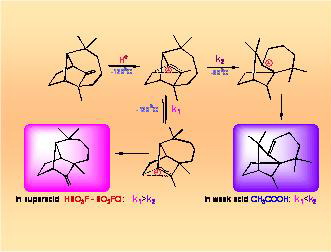
The conducted research has made it possible to lay down the foundation for quantitative theory of molecular rearrangements proceeding with formation of carbocation intermediates and to demonstrate the possibility to forecast the main directions of multi-route rearrangements of natural terpene compounds. It has been established that nonclassical carbocations play a vital role in cationoid organic reactions. In one of the broadest areas of organic chemistry it has been made possible for the first time to move from gathering qualitative data to their interpretation on a quantitative level on the basis of fundamental principles of physical organic chemistry.
There have been obtained extensive data on the ability of various carbocations to chemical transformations which opened new possibilities for fine organic synthesis.
The obtained results have given a strong impetus to the further development and deepening of studies in the field of physical organic chemistry – one of the most topical fields of modern chemistry.
|
|
|
|
|
|
|
|
|
|
|
|
In 1990 Prof. V.A. Barkhash, Acad. V.A. Koptyug, Prof. V.D. Shteingarts
and Prof. V.G. Shubin, the authors of this series of studies,were awarded the Lenin Prize.
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry,
Siberian Branch of the Russian Academy of Sciences
9, Acad. Lavrentiev Ave, 630090, Novosibirsk, 90, Russsia
Prof. V.G. Shubin, Head of the Laboratory
Tel.: (383) 330-76-51. Fax: (383) 330-97-52. E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.