В журнале Materials, 2019, V. 12, N 5, Pp. 688-707 (IF 2,467) опубликован обзор с участием сотрудников Института Mariya Edeleva, Elena Bagryanskaya
Smart Control of Nitroxide-Mediated Polymerization Initiators’ Reactivity by pH, Complexation with Metals, and Chemical Transformations
Mariya Edeleva, Gerard Audran, Sylvain Marque and Elena BagryanskayaFirst published: 19 February 2019
https://doi.org/10.3390/ma12050688
Abstract
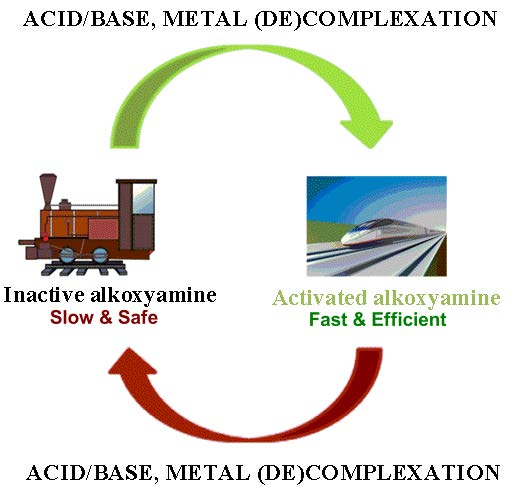
Because alkoxyamines are employed in a number of important applications, such as nitroxide-mediated polymerization, radical chemistry, redox chemistry, and catalysis, research into their reactivity is especially important. Typically, the rate of alkoxyamine homolysis is strongly dependent on temperature. Nonetheless, thermal regulation of such reactions is not always optimal. This review describes various ways to reversibly change the rate of C–ON bond homolysis of alkoxyamines at constant temperature. The major methods influencing C–ON bond homolysis without alteration of temperature are protonation of functional groups in an alkoxyamine, formation of metal–alkoxyamine complexes, and chemical transformation of alkoxyamines. Depending on the structure of an alkoxyamine, these approaches can have a significant effect on the homolysis rate constant, by a factor of up to 30, and can shorten the half-lifetime from days to seconds. These methods open new prospects for the application of alkoxyamines in biology and increase the safety of (and control over) the nitroxide-mediated polymerization method. View Full-Text
Альметрики:


