На сайте журнала Dalton Transactions (IF 4,052) опубликована статья с участием сотрудников Института д.х.н. И.И. Олейника (внс ЛЭАСМ), к.х.н. И.В. Олейник (снс ЛЭАСМ):
Probing the effect of ortho-cycloalkyl ring size on activity and thermostability in cycloheptyl-fused N,N,N-iron ethylene polymerization catalysts
Abstract
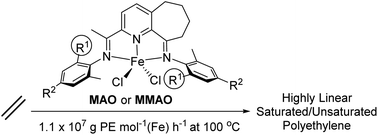
The syntheses of six bis(imino)-5,6,7,8-tetrahydrocycloheptapyridine-iron(II) chloride complexes, [2-{(Ar)NCMe}-9-{N(Ar)}C10H10N]FeCl2 (Ar = 2-(C5H9)-6-MeC6H3Fe1, 2-(C6H11)-6-MeC6H3Fe2, 2-(C8H15)-6-MeC6H3Fe3, 2-(C5H9)-4,6-Me2C6H2Fe4, 2-(C6H11)-4,6-Me2C6H2Fe5, 2-(C8H15)-4,6-Me2C6H3 Fe6), are reported in which the ring size of the ortho-cycloalkyl group has been varied as has the type of para-substituent. The molecular structures of Fe3 and Fe6 reveal square pyramidal geometries at iron while the ortho-cyclooctyl rings adopt boat-chair conformations. On treatment with either methylaluminoxane (MAO) or modified methylaluminoxane (MMAO), all six complexes showed optimal activities at 80 °C [up to 1.9 × 107 g of PE per mol Fe per h for Fe5/MMAO] for ethylene polymerization forming linear polyethylene (Tm's > 126 °C). Notably, the catalytic activities showed a marked correlation with the ring size of the ortho-cycloalkyl substituent: cyclohexyl (Fe2 and Fe5) > cyclooctyl (Fe3 and Fe6) > cyclopentyl (Fe1 and Fe4) for either para-substituent, H or Me. Furthermore, this family of iron catalysts exhibited remarkable thermostability by remaining highly active even at temperatures as high as 100 °C (1.1 × 107 g of PE per mol Fe per h); the wide variation in polymer molecular weights (Mw: 2.4–166 kg mol−1), influenced through choice of precatalyst and co-catalyst as well as by temperature and pressure, further highlights the versatility of these catalysts.
Альметрики:


