В журнале Crystal Growth & Design (IF=4,089) опубликована статья с участием сотрудников Института к.х.н. Е.А. Чулановой н.с, ЛГеТС), Е.А. Радюш (мнс, ЛОЭ), к.х.н. И.К. Шундриной (снс, ЛЭАСМ), д.х.н. И.Ю. Багрянской (внс, рук. группы ГРСА), к.х.н. Н.А. Семёнова (снс, ЛГеТС) и д.х.н. А.В. Зибарева (завлаб, ЛГеТС)
Lewis Ambiphilicity of 1,2,5-Chalcogenadiazoles for Crystal Engineering: Complexes with Crown Ethers
Elena A. Chulanova, Ekaterina A. Radiush, Inna K. Shundrina, Irina Yu. Bagryanskaya, Nikolay A. Semenov, Jens Beckmann, Nina P. Gritsan, and Andrey V. ZibarevCryst. Growth Des. 2020, 20, 9, 5868–5879
Publication Date:July 13, 2020
https://doi.org/10.1021/acs.cgd.0c00536
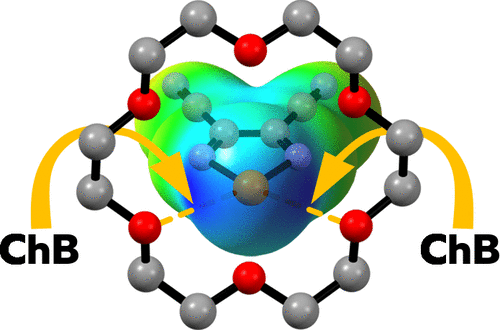
Abstract
The cocrystallization of 1,2,5-chalcogenadiazoles (chalcogen E = S, Se, and Te) with cyclic polyethers 18-crown-6 (18-c-6) and dibenzo-18-crown-6 (db-18-c-6) yielded the molecular complexes characterized by X-ray diffraction and thermogravimetry/differential scanning calorimetry techniques together with density functional theory (DFT) calculations and quantum theory of atoms in molecule and natural bond orbitals analysis. The complexes are bound by multiple secondary bonding interactions, the most important of which reflects the Lewis ambiphilicity of 1,2,5-chalcogenadiazoles and includes charge transfer from O atoms of the ethers onto E atoms of the chalcogenadiazoles (i.e., chalcogen bonding), and the back-donation from E to O. For complexes of 18-c-6, the DFT-calculated energies of bonding interactions correlate with the melting temperatures of the complexes, as well as with the atomic number of E and the size of the E-associated σ-holes but not with the maximum of molecular electrostatic potential at the σ-holes. Taking into account the previous results, the Lewis ambiphilicity of 1,2,5-chalcogenadiazoles may be used for applications in crystal engineering.
Альметрики:


