В журнале Journal of Photochemistry and Photobiology B: Biology (IF=4.383) опубликована статья с участием сотрудников Института: к.х.н. И.А. Кирилюка (завлаб ЛАС) и д.ф.-м.н., проф. Е.Г. Багрянской (директор НИОХ, завлаб ЛМР)
Application of EPR to porphyrin-protein agents for photodynamic therapy
Natalya E.Sannikova, Ivan O. Timofeev, Alexey S. Chubarov, Natalya Sh. Lebedeva, Aleksandr S. Semeikin, Igor A. Kirilyuk, Yuri P. Tsentalovich, Matvey V. Fedin, Elena G. Bagryanskaya, Olesya A.Krumkacheva
Journal of Photochemistry and Photobiology B: Biology, 2020, V. 211, 112008
https://doi.org/10.1016/j.jphotobiol.2020.112008
Abstract
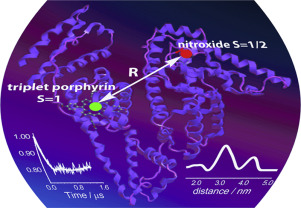
Recently, a new type of spin labels based on photoexcited triplet molecules was proposed for nanometer scale distance measurements by pulsed dipolar electron paramagnetic resonance (PD EPR). However, such molecules are also actively used within biological complexes as photosensitizers for photodynamic therapy (PDT) of cancer. Up to date, the idea of using the photoexcited triplets simultaneously as PDT agents and as spin labels for PD EPR has never been employed. In this work, we demonstrate that PD EPR in conjunction with other methods provides valuable information on the structure and function of PDT candidate complexes, exemplified here with porphyrins bound to human serum albumin (HSA). Two distinct porphyrins with different properties were used: amphiphilic meso-tetrakis(4-hydroxyphenyl)porphyrin (mTHPP) and water soluble cationic meso-tetrakis(N-methyl-4-pyridyl)porphyrin (TMPyP4); HSA was singly nitroxide-labeled to provide a second tag for PD EPR measurements. We found that TMPyP4 locates in a cavity at the center of the four-helix bundle of HSA subdomain IB, close to the interface with solvent, thus being readily accessible to oxygen. As a result, the photolysis of the complex leads to photooxidation of HSA by generated singlet oxygen and causes structural perturbation of the protein. Contrary, in case of mTHPP porphyrin, the binding occurs at the proton-rich pocket of HSA subdomain IIIA, where the access of oxygen to a photosensitizer is hindered. Structural data of PD EPR were supported by other EPR techniques, laser flash photolysis and protein photocleavage studies. Therefore, pulsed EPR on complexes of proteins with photoexcited triplets is a promising approach for gaining structural and functional insights into such PDT agents.
Альметрики:


