В журнале The Journal of Organic Chemistry, (IF 4,354I) опубликована статья с участием сотрудников Института - к.х.н.М.С. Казанцева (завлаб ЛОЭ) и к.х.н. А.А. Сониной (снс, ЛОЭ):
P2O5-Promoted Cyclization of Di[aryl(hetaryl)methyl] Malonic Acids as a Pathway to Fused Spiro[4.4]nonane-1,6-Diones
Konstantin S. Ivanov, Tim Riesebeck, Alexandrina Skolyapova, Irina Liakisheva, Maxim S. Kazantsev , Alina A. Sonina, Roman Yu Peshkov, and Evgeny A. MostovichJ. Org. Chem. 2022, 87, 5, 2456–2469,
Publication Date (Web):February 15, 2022
https://doi.org/10.1021/acs.joc.1c02379
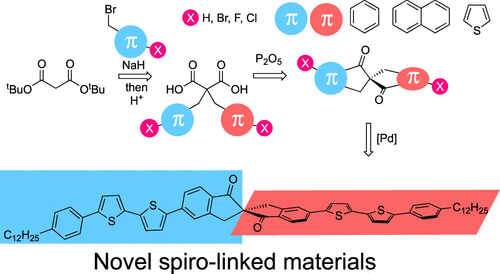
Abstract
Conventional spiro-linked conjugated materials are attractive for organic optoelectronic applications due to the unique combination of their optical and electronic properties. However, spiro-linked conjugated materials with conjugation extension directed along the main axis of the molecule are still only rare examples among the vast number of spiro-linked conjugated materials. Herein, the synthesis, leading to π-extended spiro-linked conjugated materials─spiro[4.4]nonane-1,6-diones and spiro[5.5]undecane-1,7-diones─has been developed and optimized. The proposed design concept starts from readily available malonic esters and contains several steps: double alkylation of malonic ester with bromomethylaryl(hetaryl)s; conversion of a malonic ester into the corresponding malonic acid; electrophilic spirocyclization of the latter into the annulated spiro[4.4]nonane-1,6-dione or spiro[5.5]undecane-1,7-dione in the presence of phosphorus pentoxide. On the basis of these insights, the developed method yielded spiro-linked conjugated cores fused with benzene, thiophene, and naphthalene, decorated with active halogen atoms. The structures of the synthesized spirocycles were determined by single-crystal X-ray diffraction analysis. Benzene fused spiro[4.4]nonane-1,6-dione decorated with bromine atoms was transformed into V-shape phenylene-thiophene co-oligomer type spirodimers via Stille coupling. The spiro-bis(4-n-dodecylphenyl)-2,2′-bithiophene derivative possessed high photoluminescence properties in both solution and solid state with a photoluminescence quantum yield (PL QY) of 38%.
Альметрики:


