В журнале Organic & Biomolecular Chemistry (IF 3,89) опубликована статья с участием сотрудников Института снс(к.х.н.) А.М. Генаева и снс Г.Е. Сальникова
DFT insights into superelectrophilic activation of α,β-unsaturated nitriles and ketones in superacids
Alexander M. Genaev, George E. Salnikov and Konstantin Yu. Koltunov
Org. Biomol. Chem., 2022,20, 6799-6808,
first published on 06 Aug 2022
https://doi.org/10.1039/D2OB01141G
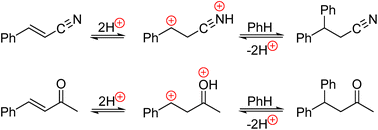
Abstract
Superelectrophilic activation of α,β-unsaturated carbonyl compounds and their isoelectronic analogs, proceeding normally under superacidic conditions, have led to a great variety of beneficial synthetic transformations. However, the essence of such activation is not yet fully recognized, while a number of alternative views on the subject have been discussed at length in the literature. Here, taking the example of virtual reactions of cinnamonitrile and benzalacetone with benzene, their feasible mechanistic variants, including multiple protonation (coordination to AlCl3) of the reactants, were analyzed based on density functional theory (DFT). It is revealed that the most plausible reaction pathways involve the initial N- or O-protonation (coordination to AlCl3) of the activated compounds followed by subsequent protonation on the α-C-atom. Dicationic superelectrophiles thus formed ensure practically barrier-free reactions with benzene in addition to a more favorable energetic profile of their generating, which is in marked contrast to other potential reaction pathways.
Альметрики:


