На сайте журнала Organic & Biomolecular Chemistry (IF 3,89) опубликована статья сотрудников Института
снс(к.х.н.) А.В. Шернюкова, снс Г.Е. Сальникова, нс(к.х.н.) В.И. Краснова и снс(кхн) А.М. Генаев
Cluster halogenation of adamantane and its derivatives with bromine and iodine monochloride
Andrey V. Shernyukov, George E. Salnikov, Vyacheslav I. Krasnov and Alexander M. Genaev
Org. Biomol. Chem., 2022, Advance Article, first published on 18 Oct 2022
Abstract
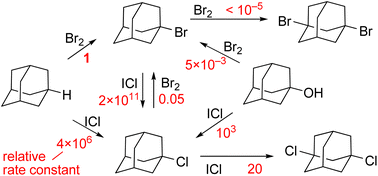
Noncatalytic halogenation of adamantane (AdH) with bromine or iodine monochloride was found to proceed according to the cluster mechanism featuring high kinetic order with respect to the halogen and a sharp decrease in the calculated energy barrier when additional halogen molecules are involved in the quantum chemical system. In the reaction with Br2, 1-AdBr formed selectively. This reaction proved to be first order in terms of AdH and approximately seventh order in Br2, and its rate does not depend on the rising concentration of HBr. It was demonstrated that the reaction of AdH with ICl is sixth order in ICl, and at the first stage, 1-AdCl forms. According to kinetic data, this reaction requires 3 equivalents of ICl. The rate of 1-AdCl chlorination leading to the 1,3-di-Cl derivative turned out to be 105 times slower than that of AdH. The halogen exchange reaction of 1-AdBr with ICl yielded 1-AdCl, and this reaction is fast and is first order in ICl. Another halogen exchange reaction, AdCl + Br2 = AdBr + BrCl, proceeded much more slowly, and the equilibrium is strongly shifted to the left (equilibrium constant: 10−6). With an excess of either Br2> or ICl, adamantanol (1-AdOH) was found to enter into a slow (compared to AdH) exchange reaction producing 1-AdBr or 1-AdCl, respectively. In all the studied reactions, ICl is ∼106-fold more active than Br2. According to DFT data, the reactions of AdH with Br2 and ICl have similar rate-limiting stages, where the H atom from AdH and X atom from polarized halogen cluster X2n> move toward each other forming an HX molecule and ion pair Ad<+X2n−1−.
Альметрики: