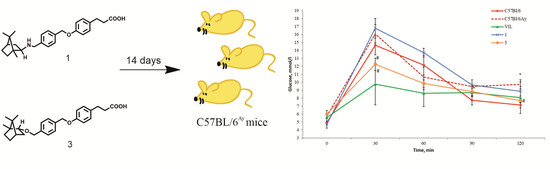
В престижном журнала The Journal of the American Chemical Society (JACS) (IF 15,419) опубликована статья с участием сотрудников Института д.х.н. И.Ю. Багрянской (внс, рук. группы ГРСА), к.х.н. И.К. Шундриной (снс, ЛМР, рук. группы ГТ) и к.х.н. М.C. Казанцева (завлаб ЛОЭ):
Platform for High-Spin Molecules: A Verdazyl-Nitronyl Nitroxide Triradical with Quartet Ground State
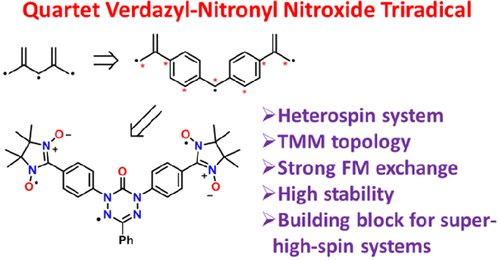
Получен и структурно охарактеризован гетероспиновый квартетный трирадикал, содержащий оксовердазил и два нитронилнитроксильных фрагмента. Трирадикал устойчив на воздухе и обладает высокой термической стабильностью в инертной атмосфере. Данные магнитных измерений указывают на достаточно сильные внутримолекулярные обменные ферромагнитные взаимодействия между парамагнитными центрами. Полученный гетероспиновый трирадикал с квартетным основным состоянием может служить платформой для получения дендримерных высокоспиновых систем.
Abstract
Thermally resistant air-stable organic triradicals with a quartet ground state and a large energy gap between spin states are still unique compounds. In this work, we succeeded to design and prepare the first highly stable triradical, consisting of oxoverdazyl and nitronyl nitroxide radical fragments, with a quartet ground state. The triradical and its diradical precursor were synthesized via a palladium-catalyzed cross-coupling reaction of diiodoverdazyl with nitronyl nitroxide-2-ide gold(I) complex. Both paramagnetic compounds were fully characterized by single-crystal X-ray diffraction analysis, superconducting quantum interference device magnetometry, EPR spectroscopy in various matrices, and cyclic voltammetry. In the diradical, the verdazyl and nitronyl nitroxide centers demonstrated full reversibility of redox process, while for the triradical, the electrochemical reduction and oxidation proceed at practically the same redox potentials, but become quasi-reversible. A series of high-level CASSCF/NEVPT2 calculations was performed to predict inter- and intramolecular exchange interactions in crystals of di- and triradicals and to establish their magnetic motifs. Based on the predicted magnetic motifs, the temperature dependences of the magnetic susceptibility were analyzed, and the singlet–triplet (135 ± 10 cm–1) and doublet-quartet (17 ± 2 and 152 ± 19 cm–1) splitting was found to be moderate. Unique high stability of synthesized verdazyl-nitronylnitroxide triradical opens new perspectives for further functionalization and design of high-spin systems with four or more spins.
Альметрики:











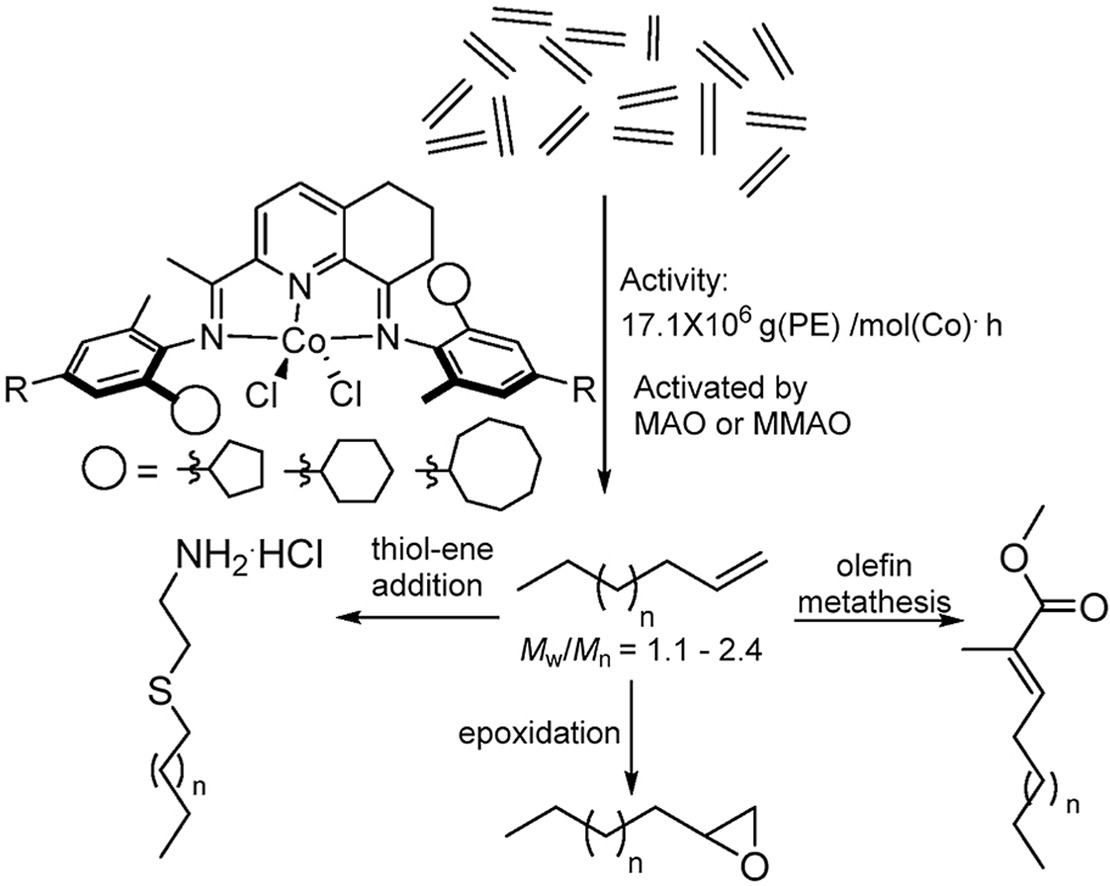
 CCH3)-8-(ArN)-5,6,7-C9H8N]CoCl2 (Ar = 2-(C5H9)-6-MeC6H3 Co1, 2-(C6H11)-6-MeC6H3 Co2, 2-(C8H15)-6-MeC6H3 Co3, 2-(C5H9)-4,6-Me2C6H2 Co4, 2-(C6H11)-4,6-Me2C6H2 Co5, 2-(C8H15)-4,6-Me2C6H2 Co6), distinguishable by the ring size of the ortho-cycloalkyl substituent and type of para-R group, have been synthesized and characterized. A distorted square pyramidal geometry is a feature of the molecular structure of Co4 with the two ortho-cyclopentyl groups located on neighboring N-aryl groups trans-configured. Compounds Co1 – Co6, on activation with methylaluminoxane (MAO) or modified MAO (MMAO), proved highly productive catalysts for ethylene polymerization at 60 °C [up to 17.1 × 106 g (PE) mol−1(Co) h−1 for cyclopentyl-containing Co4/MAO]; even at 90 °C significant activity was attainable (up to 6.75 × 106 g (PE) mol−1(Co) h−1). Strictly linear polyethylene waxes of low molecular weight (ca. 1.50 kg mol−1), narrow dispersity (Mw/Mn range: 1.1–2.4) and incorporating high levels of vinyl end-groups were generated. Post-functionalization of these PE waxes by epoxidation, thiol-ene addition and cross-olefin metathesis to form e-PE, PE-S-CH2CH2NH2·HCl and PE-MMA, respectively, has been demonstrated. For comparative purposes, [2-(ArN
CCH3)-8-(ArN)-5,6,7-C9H8N]CoCl2 (Ar = 2-(C5H9)-6-MeC6H3 Co1, 2-(C6H11)-6-MeC6H3 Co2, 2-(C8H15)-6-MeC6H3 Co3, 2-(C5H9)-4,6-Me2C6H2 Co4, 2-(C6H11)-4,6-Me2C6H2 Co5, 2-(C8H15)-4,6-Me2C6H2 Co6), distinguishable by the ring size of the ortho-cycloalkyl substituent and type of para-R group, have been synthesized and characterized. A distorted square pyramidal geometry is a feature of the molecular structure of Co4 with the two ortho-cyclopentyl groups located on neighboring N-aryl groups trans-configured. Compounds Co1 – Co6, on activation with methylaluminoxane (MAO) or modified MAO (MMAO), proved highly productive catalysts for ethylene polymerization at 60 °C [up to 17.1 × 106 g (PE) mol−1(Co) h−1 for cyclopentyl-containing Co4/MAO]; even at 90 °C significant activity was attainable (up to 6.75 × 106 g (PE) mol−1(Co) h−1). Strictly linear polyethylene waxes of low molecular weight (ca. 1.50 kg mol−1), narrow dispersity (Mw/Mn range: 1.1–2.4) and incorporating high levels of vinyl end-groups were generated. Post-functionalization of these PE waxes by epoxidation, thiol-ene addition and cross-olefin metathesis to form e-PE, PE-S-CH2CH2NH2·HCl and PE-MMA, respectively, has been demonstrated. For comparative purposes, [2-(ArN
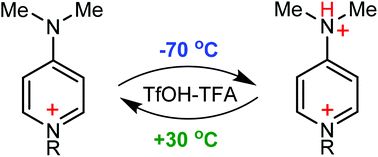


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(O)–tert-Bu coupler) was investigated under various conditions. It was found that prolongation of reaction time caused transformation of the initial diradical into new diradicals with the unique >C
N(O)–tert-Bu coupler) was investigated under various conditions. It was found that prolongation of reaction time caused transformation of the initial diradical into new diradicals with the unique >C