The paper of NIOCh's researchers is published in the journal European Journal of Organic Chemistry (IF 3.029)
The Diels–Alder Reaction for the Synthesis of Polycyclic Aromatic Compounds

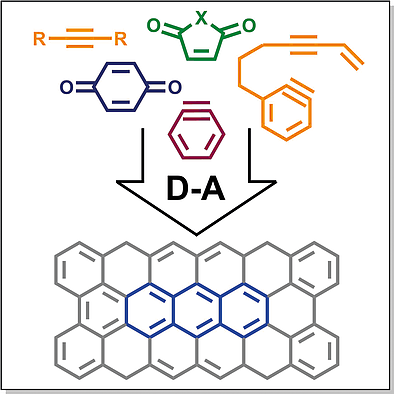
Abstract
Polycyclic aromatic compounds (PACs) represent a class of molecules consisting of two or more condensed aromatic rings. They are currently of great interest as potential semiconducting materials for organic field‐effect transistors, light‐emitting diodes, and solar panels. Substituted PACs are not always readily available and may require complicated multi‐step synthetic procedures to be obtained. One of the promising methods of the synthesis of functionalized PACs is the assembly of polycyclic backbones from substituted building blocks via cycloaddition reactions, in particular, the Diels–Alder reaction. In this minireview we aimed to cover the recent progress in the application of the Diels–Alder reaction in PACs synthesis with the emphasis on the structures of dienophiles. While quinones and arynes still act most frequently as the dienophilic components, other dienophiles with activated double and triple carbon–carbon bonds attract attention due to the ability of insertion of functional groups into polycyclic structures. In the recent decade variants of Dehydro Diels–Alder reaction, especially HexaDehydro Diels–Alder reaction (HDDA) attract much attention leading to the formation of polycyclic structures in a single step. The present review covers mostly papers published during the past decade; older works are referenced only when it is necessary for the understanding of the results discussed.
Altmetrics:


