The paper of NIOCh's researchers (international collaboration) is published in the journal CrystEngComm, 2019, V. 21, N 39, Pp 5931-5946 (IF 3,382)
Impact of molecular packing rearrangement on solid-state fluorescence: polyhalogenated N-hetarylamines vs. their co-crystals with 18-crown-6
Tamara A. Vaganova, Yurij V. Gatilov, Enrico Benassi, Igor P. Chuikov, Denis P. Pishchur and Evgenij V. MalykhinCrystEngComm, 2019, V. 21, N 39, Pp 5931-5946
First published: 23 August 2019
https://doi.org/10.1039/C9CE00645A
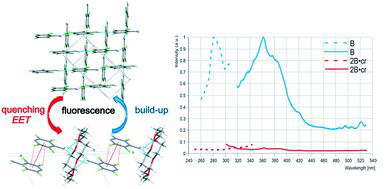
Abstract
A series of polyhalogenated hetarylamines and their co-crystals with 18-crown-6 ether was used to reveal the effect of co-crystallization on the supramolecular structure and fluorescence properties as well as the relationship between these characteristics and peculiarities of the hetarylamine chemical structure (α- or γ-position of the NH2 group, number of Cl and F substituents, pyridine or quinoline framework, presence of a fluorophore fragment). Incorporation of crown ether into the crystal matrix of the hetarylamine results in a significant rearrangement of the supramolecular structure. In all cases, the N–H⋯Nhet and N–H⋯F H-bonds are replaced by the N–H⋯Ocrown bond. Changes in π-electron interactions that affect fluorescence characteristics depend on the amine structure. In the co-crystals of γ-aminopyridines, replacement of C–F⋯πF interactions with πF⋯πF stacking causes the fluorescence quenching or a bathochromic shift of λem, whereas replacement of πF⋯πF or πF⋯πH stacking with C–F⋯πF interactions or πH⋯πH stacking in the co-crystals of α-aminopyridines is accompanied by the hypsochromic shift of λem. γ-Aminopyridines show more pronounced changes in fluorescence characteristics as compared to α-aminopyridines, the effect being dependent on the nature of the halogen substituents. The fluorescence properties of aminoquinolines, regardless of the amino group location, are insignificantly modified due to the ability of the extended π-system to minimize interaction changes. Exploration of the nature of the excited states of isolated molecules, homocrystals and co-crystals using quantum mechanical calculations evidences that fluorescence quenching occurs due to EET, facilitated by the presence of crown ether molecules. The bathochromic and hypsochromic shifts of emission are caused by the change in a mutual orientation of the hetarylamine molecules with subsequent different stabilization of the ground and excited states. Using DSC, the co-crystals' supramolecular structure was found to self-organize from solution or melt and to regenerate in the melting–crystallization cycle. The revealed modification of the hetarylamines' fluorescence properties due to the formation of co-crystals with crown ether seems to be promising for use in solid-state supramolecular chemo and thermo sensors (indicators).
Altmetrics:


