The paper of NIOCh's researchers (with international collaboration) is published in the journal The Journal of Organic Chemistry, (IF 4,805I)
Acid-Catalyzed Versus Thermally Induced C1–C1′ Bond Cleavage in 1,1′-Bi-2-naphthol: An Experimental and Theoretical Study
Alexander M. Genaev, Lyudmila N. Shchegoleva, George E. Salnikov, Andrey V. Shernyukov, Leonid A. Shundrin, Inna K. Shundrina, Zhongwei Zhu, Konstantin Yu. KoltunovJ. Org. Chem. 2019, 84, 11, 7238-7243
First published: 14 May 2019
https://doi.org/10.1021/acs.joc.9b00915
Abstract
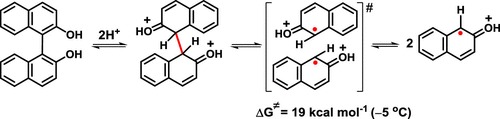
Experiments show that 1,1′-bi-2-naphthol (BINOL) undergoes facile C1–C1′ bond cleavage under action of triflic acid at temperatures above 0 °C to give mainly 2-naphthol along with oligomeric material. CASSCF and MRMP//CASSCF computations have demonstrated unambiguously that this unusual mode of scission of the biaryl bond can occur in the C1,C1′-diprotonated form of BINOL via a mechanism involving homolytic cleavage prompted by the intramolecular electrostatic repulsion. These findings also provide insights into the mechanism of a comparatively easy thermal cleavage of BINOL, implying the intermediacy of its neutral diketo form.
Altmetrics:


