The article with the participation of NIOCh's researchers is published in the journalу Organic & Biomolecular Chemistry (IF 3,412)
Unusual temperature-sensitive protonation behaviour of 4-(dimethylamino)pyridine
Alexander M. Genaev, George E. Salnikov and Konstantin Yu. Koltunov
Org. Biomol. Chem., 2021,19, 866-872
This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC
https://doi.org/10.1039/D0OB01893G
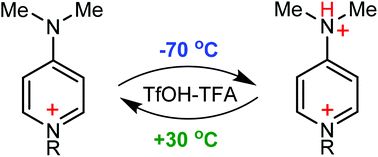
Abstract
Stimuli-responsive and, in particular, temperature-responsive smart materials have recently gained much attention in a variety of applications. On the other hand, 4-(dimethylamino)pyridine (DMAP) and related structures are widely used as nucleophilic catalysts and also as specific parts of rationally designed molecules, where reversible reactions of the pyridinic nitrogen with electrophiles are involved. In our study, we have found an unexpectedly significant impact of temperature on the protonation degree of DMAP derivatives, especially in the case of protonation of the 4-(dimethylamino)-1-(2,3,5,6-tetrafluoropyridin-4-yl)pyridinium cation, derived from the reaction of DMAP with pentafluoropyridine. Thus, when dissolved in the TfOH-SO2ClF-CD2Cl2 acid system at 30 °C, this cation underwent a slight (<7%) protonation on the dimethylamino group, while the temperature decrease to −70 °C resulted in its complete protonation. Notably, such a scale of this phenomenon has never been observed before for other weak nucleophiles, being many times lower at the same change of temperature. The mechanistic aspects of these intriguing results are discussed.
Altmetrics:


