The article with participation of NIOCh's researchers ( I.V.Il'ina, N.S.Li-Zhulanov, D.V.Korchagina, O.V.Ardashov, K.P.Volcho, N.F.Salakhutdinov) is published in the journal Applied Catalysis A: General (IF=5.006)
Catalytic synthesis of terpenoid-derived hexahydro-2H-chromenes with analgesic activity over halloysite nanotubes
A.Yu.Sidorenko, Yu.M.Kurban, I.V.Il'ina, N.S.Li-Zhulanov, D.V.Korchagina, O.V.Ardashov, J.Wärnå, K.P.Volcho, N.F.Salakhutdinov, D.Yu.Murzin, V.E.Agabekov
Applied Catalysis A: General
Volume 618, 25 May 2021, 118144
https://doi.org/10.1016/j.apcata.2021.118144
Abstract
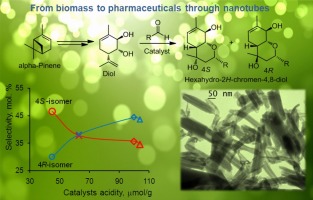
Condensation of α-pinene derived p-menta-1,8-diene-5,6-diol (diol) with decanal was studied for the first time over modified halloysite nanotubes (HNT). The yield of the desired hexahydro-2H-chromene-4,8-diol with analgesic activity was 76–80 % practically not depending on the catalyst type, while selectivity to 4S-isomer decreased, and to 4R-isomer increased with increasing acidity. The highest selectivity to 4S-diastereomer (48.1 %) on halloysite is a result of weak acidity of this catalyst. DFT optimization of the key intermediate structure shows that the nucleophile attack proceeds at the equatorial position with the 4S-diastereomer formation, which was preferred on halloysite. On strong Brønsted (Amberlyst-15) and Lewis (scandium triflate) acids the target product yield did not exceed 37 % because of dehydration. Halloysite nanocatalysts displayed a stable performance. In the case of diol reaction with a set of carbonyl compounds, the yields of hexahydro-2H-chromene-4,8-diols (up to 88.0%) and the ratio of its 4S/4R isomers (up to 21.0) were significantly higher than on other catalysts.
Альметрики:


