The article with participation of NIOCh's researchers is published in
Physical Chemistry Chemical Physics, (IF 3,676)
Impact of fluorination and chlorination on the electronic structure, topology and in-plane ring normal modes of pyridines
Enrico Benassi, Tamara Vaganova, Evgenij Malykhin and Haiyan FanPhysical Chemistry Chemical Physics,
2021, V. 23, N. 34, Pp.18958-18974
doi: 10.1039/D1CP02342J
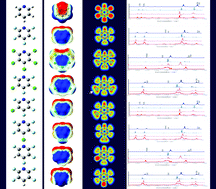
Abstract
Seven partially and fully fluorinated/chlorinated pyridines were investigated by means of FT-IR and Raman spectroscopy combined with quantum chemical calculations, mainly aiming to detect how the nature and position of F and Cl substituents affect the in-plane ring normal modes (RNMs) of pyridines in terms of vibrational wavenumbers, force constants, IR intensities and Raman activities. Taking pyridine as the reference, the RNMs and some derived RNMs through coupling with related C–X (X = F, Cl) stretching vibrations were identified on the basis of their composition in terms of internal coordinates. The impact of fluorination and chlorination on these RNMs was also discussed from the perspective of frontier molecular orbitals (MOs), maps of the molecular electrostatic potential (MEP) and the molecular topology. Natural bond orbital (NBO) analysis revealed the consequences of substitutions on the intramolecular charge delocalisation and consequently the ring bond strength. Moreover, the effects of anharmonicity of the potential on vibrational frequencies were presented and discussed.
Альметрики:


