INTERMEDIATE PRODUCTS FOR THE SYNTHESIS OF MODERN AND PERSPECTIVE PYRETHROID INSECTICIDES
Research conducted in 1989-1994 under the supervision of A.V.Tkachev; State Prize for Young Scientists, 1995 and 1996; Gold Medal of the 44th International Exhibition of Inventions, Research and Industrial Innovations, BRUSSELS EURECA'95 (1995).
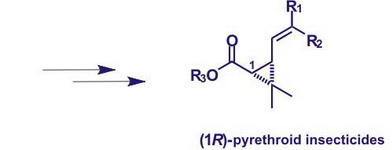
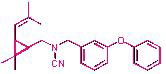
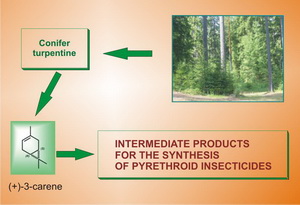 Pyrethroid insecticides are the most effective birth control means of arthropoda which insectoracaricide activity largely depends on the structure of the molecules. Production of (1R)-configuration pyrethroid insecticides in enantiomeric pure form makes it possible to increase significantly their biological activity and decrease environmental damage of their application. One of the most perspective approaches to the production of optically active pyrethroids is based on the use of natural chiral compounds as initial products for their synthesis. Natural monoterpene hydrocarbon (+)-3-carene – a turpentine component obtained in the processing of many conifers (family Pinaceae) – is one of such substances.
Pyrethroid insecticides are the most effective birth control means of arthropoda which insectoracaricide activity largely depends on the structure of the molecules. Production of (1R)-configuration pyrethroid insecticides in enantiomeric pure form makes it possible to increase significantly their biological activity and decrease environmental damage of their application. One of the most perspective approaches to the production of optically active pyrethroids is based on the use of natural chiral compounds as initial products for their synthesis. Natural monoterpene hydrocarbon (+)-3-carene – a turpentine component obtained in the processing of many conifers (family Pinaceae) – is one of such substances.
The conducted research has made it possible to develop a new approach – instead of traditional oxidation systems – to the construction of molecules of intermediate products for the synthesis on the basis of (+)-3-carene pyrethroid insecticides in optically pure form:
 |
|
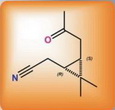
The new approach is based on the reaction of nitrosochlorination of carene for disintegration of a six-membered carbocycle of the initial molecule instead of traditional oxidation reactions. Available synthetic methods of obtaining target compounds have been developed, which opened up new routes for the production of derivatives from (1R)-cyclopropanecarboxylic acids with the use of rearrangements running in mild conditions at nitrogen atom (Beckman, Hofman, Timan).
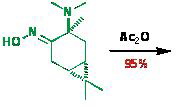
The main idea of the new synthetic approach is transformation of oxime 3-(N,N-dimethylamine)-carane-4-on to ketonitrile with almost quantitative yield in the action of acetic anhydride, with the target product being easily isolated from the reaction by-products. The given method can be easily scaled and produced at the pilot plant of the Novosibirsk Institute of Organic Chemistry:
 |
|
|
 |
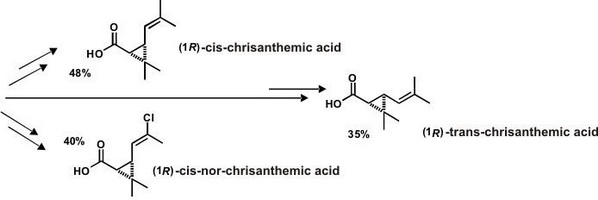
Pyrethroid esters on the basis of (1R)-trans-chrysanthemic acid are characterized by the combination of high biological activity with very low toxicity for mammals, which makes them extremely perspective as indoor domestic insecticides. In general case, pyrethroid esters of (1R)-trans-chrysanthemic acid exceed the esters of (1R)-cis-chrisanthemic acid in insecticide activity; however, (1R)-cis-chrisanthemates appear to be more active in respect of some objects (e.g.: mosquitos). Therefore, the development of convenient synthetic methods of (1R)-cis-chrisanthemic acids is of special interest. (1R)-cis-nor-chrisanthemic acid is a structural hybrid of (1R)-cis-chrisanthemic and permetrine acids. The obtained pyrethroids combine the properties of both chresanthemates and esters of permethrine acids; in terms of insecticide activity, they are closer to the correspondent derivatives of permethrine acid, but unlike the latter, they are significantly less toxic for fish, which is sure to be their prime advantage.
Simple and effective schemes of obtaining three pyrethroid acids:
 |
|
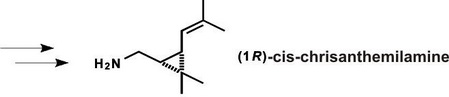
Production of nitrogen-containing analogues of pyrethroids. Hydrolysis of a molecule on the place of ester rearrangement in the action of esterases is one of the most important mechanisms of pyrethroid detoxication in the organisms of insects. There have been developed synthetic methods of (1R)-cis-chrisanthemilamine – an important intermediate compound in obtaining aza-analogues of pyrethroid insecticides, such as (1R)-cis-chrisanthemil-(3-phenoxy benzyl)-cyanamide:
 |
|
||
|
|
|||
|
|
|
|
|
Thus, the conducted research has made it possible to develop a simple, efficient and easily scalable synthetic routes which allowed us to transform natural terpene hydrocarbon (+)-3-carene with high yield to valuable biologically active substances of pyrethroid series. The results of the research work can be applied in various spheres – production of birth control pesticides, synthesis of pharmaceutical products, fine organic synthesis of chiral substrates, etc.
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry,
Siberian Branch of the Russian Academy of Sciences
9, Acad. Lavrentiev Ave, 630090, Novosibirsk, 90, Russsia
PhD. A.V. Tkachev, Head of the Laboratory
E-mail:This email address is being protected from spambots. You need JavaScript enabled to view it. Tel.: 8(383) 330-98-55 Fax: 8(383) 330-97-52