
NIOC research activity in the chemistry of organic hydroxylamine derivatives resulted in the development of an original family of stable nitroxyl radicals – imidazoline nitroxides, the derivatives of 3-imidazoline or 3-imidazoline-3-oxide. The 3-imidazoline or 3-imidazoline 3-oxide nitroxides have imine or nitrone groups along with the nitroxide moiety in the same heterocycle. Close topological proximity of imine or nitrone group and nitroxide radical center allows their reciprocal influence and determines unique chemical properties of imidazoline nitroxides. Due to specific chemical reactivity and high chemical stability imidazoline nitroxides have found numerous applications in different branches of science and technology.
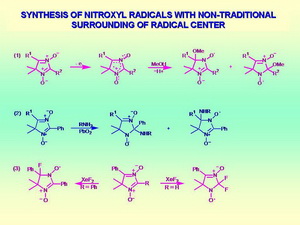
Special methodologies have been developed for the synthesis of imidazoline nitroxides, and numerous nitroxides of this family have been prepared with various functional groups to provide required physical and chemical properties and to achieve desired spectral characteristics. One of the most impressive examples to demonstrate synthetic potential of the new methodologies is the synthesis of imidazoline nitroxides with unusual surrounding of the radical center. The synthesis of these compounds is based on oxidative activation of nitrone group in the structure of heterocycle with the consequent addition of nucleophilic agent. A number of nitroxides with alkoxy, amino groups or fluorine atom at α-carbon atom of the nitroxide moiety have been prepared which differ in their properties from conventional tetraalkyl-sustituted nitroxides.
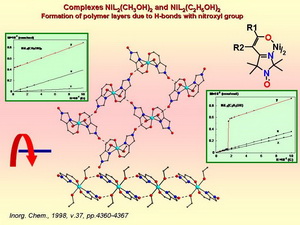
Addition of the functional group capable for coordination into position 4 of the heterocycle makes the imidazoline nitroxides efficient chelating agents because imine nitrogen in 3-imidazoline nitroxides and nitrone oxygen in 3-imidazoline-3-oxides may participate in coordination with metal ions. Strong spin exchange interactions are observed in the complexes of paramagnetic metal ions. On the basis of these complexes a new family of ferromagnetic materials has been created.
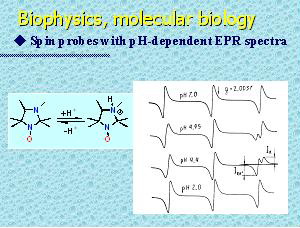
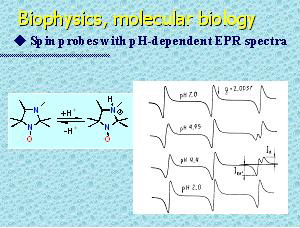
Lone pair of electrons of nitrogen atom N-3 of 3-imidazoline and imidazolidine nitroxides makes these nitroxides basic. Reversible protonation of this basic center adjacent to the nitroxide moiety produces remarkable changes in the EPR-spectrum. This phenomenon was used in the original method for pH measurement. Imidazoline and imidazolidine nitroxides are the most effective pH-sensitive spin probes. The probes available permit measurements within pH range 0-14 with accuracy up to 0.05 pH units. Nitroxides with pH-dependent EPR spectra have been successfully used as pH-sensitive spin probes for determination of local pH and for investigation of proton-related transport processes in various systems including biological objects and heterogeneous organic and inorganic materials, e.g. studies of transmembrane transport processes and surface potential of membranes and proteins, in vivo estimation of efficacy of drug-delivery systems, pH monitoring in non-transparent «water-in-oil» systems, studies of surface properties of polyelectrolites, pH-measurements in mesopores of ceolites, etc..
Imidazoline nitroxides have played an important role for the solution of various theoretical and applied problems in various fields of science. Nowadays, imidazoline nitroxides are used by many scientists for the researches in organic, physical, analytical, polymer and coordination chemistry, materials science, molecular biology, biochemistry, physiology and pharmacology.
The results of the study of stable NR are published in a large series of scientific works and summarized in scientific reviews, monographs and articles, which are well-known to the Russian and foreign scientific community.
Authors:
- Prof. L.B.Volodarsky, Prof. I.A. Grigor’ev, Prof. V.A. Reznikov (NIOC SB RAS);
- Prof. V.I. Ovcharenko, Prof. R.Z. Sagdeev (ITC SB RAS);
- Prof. S.V. Larionov (NIIC SB RAS); Prof. V.V. Khramtsov (NICK&C SB RAS).
State Prize of the Russian Federation in science and technology, 1994
N.N.Vorozhtsov Novosibirsk Institute of Organic Chemistry,
Siberian Branch of the Russian Academy of Sciences
Acad. Lavrentiev Ave., 9, Novosibirsk 90, 630090
Prof. I.A.Grigor’ev, Director
Tel.: (383) 330-88-50; >Fax: 330-97-52
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.; This email address is being protected from spambots. You need JavaScript enabled to view it.


