В журнале CrystEngComm (IF 3,545) опубликована статья с участием сотрудников Института к.х.н. Т.А. Вагановой (снс ЛГетС), д.х.н. Ю.В. Гатилова (внс ГРСА), к.х.н. И.П. Чуйкова (нс ЛМР, ГОС) и д.х.н. Е.В. Малыхина (гнс ЛГетС)
Polyhalogenated aminobenzonitriles vs their co-crystals with 18-crown-6: amino group position as a tool to control crystal packing and solid-state fluorescence
Tamara A. Vaganova, Enrico Benassi, Yurij V. Gatilov, Igor P. Chuikov, Denis P. Pishchur and Evgenij V. MalykhinCrystEngComm, 2022, 24(5), 987-1001,
First published 20 Dec 2021
https://doi.org/10.1039/D1CE01469B
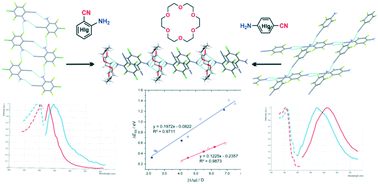
Abstract
A series of para- and ortho-aminobenzonitriles differing in the nature and number of halogen substituents were used to synthesize 2:1 co-crystals with 18-crown-6 ether. The supramolecular structure of the obtained co-crystals as well as aminobenzonitrile crystals was studied in detail using single-crystal X-ray diffraction. Incorporation of the crown ether into the crystal matrix of arylamine results in the replacement of the H-bonds between amine molecules (N–H⋯N
C and N–H⋯F) by the bond with a crown oxygen atom (N–H⋯Ocr). The crystal packing rearrangement modifies the π-electron interactions between aminobenzonitrile molecules both in the type of contact (C–F⋯π, C–Cl⋯π, C
N⋯π, π⋯π) and mutual arrangement of the stacked molecules (parallel/anti-parallel, displaced/rotated). These transformations cause a change in the solid-state fluorescence characteristics of aminobenzonitriles: co-crystallization is accompanied by a bathochromic shift of the fluorescence maximum in the case of para-isomers and by a hypsochromic shift in the case of ortho-isomers; the magnitude of this effect depends on the number of halogen substituents. Exploration of the nature of the intra- and intermolecular interactions, along with the excited states of the molecules in the gas phase, in aminobenzonitrile crystals and their co-crystals, using state-of-the-art TD-DFT calculations evidences that, depending on the NH2 group position, insertion of the crown ether causes either an increase in the change of the dipole moment upon photo excitation/emission with a subsequent increase in the Stokes shifts (para-aminobenzonitriles) or a decrease in these characteristics (ortho-aminobenzonitriles). This is consistent with the strengthening or weakening of π-electron aggregation in pairs of para- or ortho-aminobenzonitrile molecules, respectively, upon co-crystallization. A quantitative model that can clearly distinguish the different behaviours of ortho- and para-substituted molecules and provides an analytical tool of wide-ranging validity was proposed. The central importance of the mutual arrangement of the functions playing the role of the H-bond donor and acceptor was established; this finding may be exploited as a design tool to purposefully modify the molecular packing and tune the solid-state photophysical properties. Using DSC, the co-crystals' structure was found to self-organize in the same way upon crystallization from solution and from the melt and to regenerate in the melting–crystallization cycle.
Альметрики:


