ПОЗДРАВЛЯЕМ
научных сотрудников Института:
Д.О. Приму, к.х.н. А.Г. Макарова, д.х.н. И.Ю. Багрянскую, д.б.н. Д.С. Баева, к.х.н. Т.Ф. Елисееву, к.х.н. Л.В. Политанскую, д.х.н. А.Ю. Макарова, д.х.н. А.В. Зибарева
Dear Author,
We are excited to share that your research, published in ChemistrySelect is among the top 10% most downloaded papers!
Prima D.O., Makarov A.G., Bagryanskaya I.Yu., Kolesnikov A.E., Zargarova L.V., Baev D.S., Eliseeva T.F., Politanskaya L.V., Makarov A.Yu., Slizhov Yu.G., Zibarev A.V.,
Fluorine-containing n-6 and angular and linear n-6-n’ (n, n’ = 5, 6, 7) diaza-heterocyclic scaffolds assembled on benzene core in unified way,
ChemistrySelect, 2019, 4, 2383-2386.
DOI: 10.1002/slct.03970.
What this means for you: Among work published between January 2018 and December 2019, yours received some of the most downloads in the 12 months following online publication.
Your research generated immediate impact and helped to raise the visibility of ChemistrySelect.
In recognition of your work, we’re pleased to offer you a certificate of achievement.
Your co-authors can request their own certificates.
Thank you for helping to grow our profile so that work like yours is more discoverable.
Best wishes,
ChemistrySelect
Fluorine‐Containing n‐6 and Angular and Linear n‐6‐n’ (n, n’ = 5, 6, 7) Diaza‐Heterocyclic Scaffolds Assembled on Benzene Core in Unified Way
Darya O. Prima, Dr. Arkady G. Makarov, Prof. Dr. Irina Yu. Bagryanskaya, Andrey E. Kolesnikov, Leila V. Zargarova, Dr. Dmitry S. Baev, Dr. Tatiana F. Eliseeva, Dr. Larisa V. Politanskaya, Dr. Alexander Yu. Makarov, Dr. Yuri G. Slizhov, Prof. Dr. Andrey V. Zibarev
ChemistrySelect, 2019, V. 4, N 8, Pp 2383-2386
First published: 27 February 2019
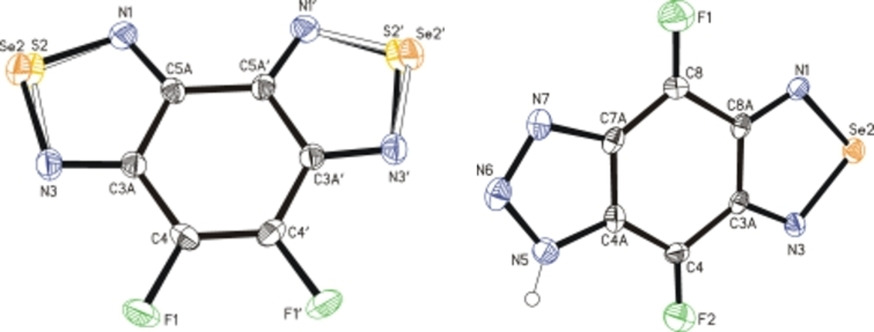
Abstract
The title compounds, including hybrids (n ≠ n’), were assembled on benzene core in a unified way based on only one starting material C6F5NH2 and a limited number of simple substitution and condensation reactions. The heterocyclic moieties were 1,3‐diazoles, 1,2,3‐triazoles, 1,2,5‐thia(selena)diazoles, 1.4‐diazines and 1,5‐diazepines, as well as 1,3,2‐dioxaboroles. Representatives of all new scaffolds were characterized by XRD. Compounds synthesized are of biomedical interest in the context of apoptotic anticancer activity, and the 1,2,5‐thiadiazole/1,3,2‐dioxaborole hybrid also in that of neutron or/and proton capture therapy of cancer. New 1,2,5‐chalcogenadiazoles are protected forms of otherwise hardly accessible fluorine‐containing di‐ and tetra‐amines for further syntheses.
Альметрики:


