Head - Cand. Sci. (Chem)
POLITANSKAYA Larisa Vladimirovna
Phone: (383) 330-91-71
internal phone: т.3-48
email:This email address is being protected from spambots. You need JavaScript enabled to view it.
Up to 2015 Laboratoty was headed by Professor, Dr. Sci. Shteingarts Vitaliy Davidovich
The laboratory has been established in 1975 as a result of transformation of the former laboratory of kinetics of homogeneous reactions (Prof. Dr. S.M. Shein).
- Research areas:
Research areas:
-
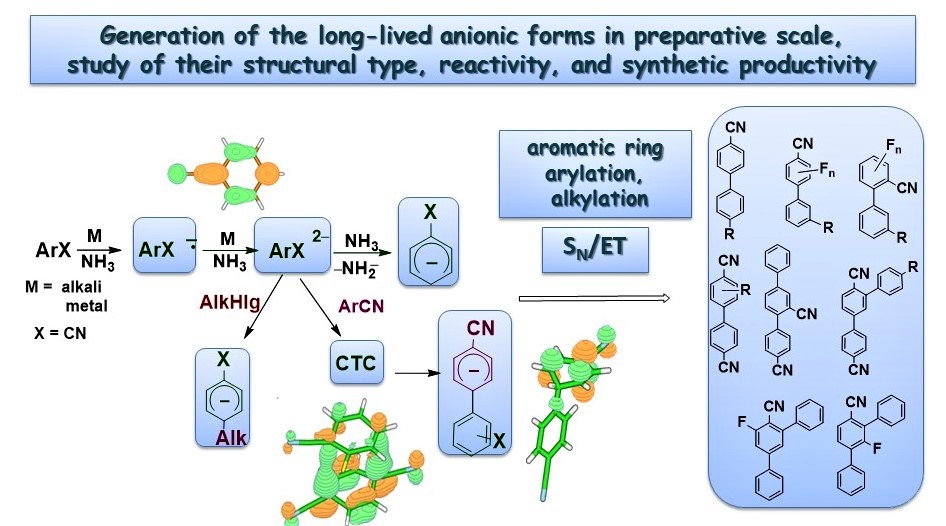
Investigation of structure, reaction mechanisms and ways of synthetic utilization of anionic forms generated by one- and two-electron reduction of aromatic compounds
-
R.Yu. Peshkov, E.V. Panteleeva, W. Chunyan, E.V. Tretyakov, V.D. Shteingarts. One-pot synthesis of 4'-alkyl-4-cyanobiaryls on the basis of the terephthalonitrile dianion and neutral aromatic nitrile cross-coupling. Beilstein Journal of Organic Chemistry, 2016, V.12, Pp 1577-1584 doi:10.3762/bjoc.12.153.
-
R.Yu. Peshkov, Ch. Wang, E.V. Panteleeva, T.V. Rybalova, E.V. Tretyakov. Radical Anions of Aromatic Carbonitriles as Reagents for Arylation of Fluorinated Benzonitriles. J. Org. Chem., 2019, 84 (2), pp 963-972 doi:10.1021/acs.joc.8b02904.
-
R.Yu. Peshkov, Ch. Wang, E.V. Panteleeva, E.V. Tretyakov. Synthesis of 4'-alkyl-[1,1'-biphenyl]-2,3'-dicarbonitriles via dimerisation of phthalonitrile radical anion in liquid ammonia. Arkivoc,2018, part vii, Pp 349-356 doi:10.24820/ark.5550190.p010.764.
-
R.Yu. Peshkov, E.V. Panteleeva, L.N. Shchegoleva, I.Yu. Bagryanskaya, T.V. Rybalova, N.V. Vasilieva, V.D. Shteingarts. Synthesis of 2-X-, 3-X-4,4'-Dicyanobiphenyls (X = CH3, OCH3, F) by Cross-Coupling of the Terephthalonitrile Dianion with Substituted Benzonitriles. European Journal of Organic Chemistry, V. 2015, N 20, pp 4524-4531, July 2015 doi:10.1002/ejoc.201500295
-
-
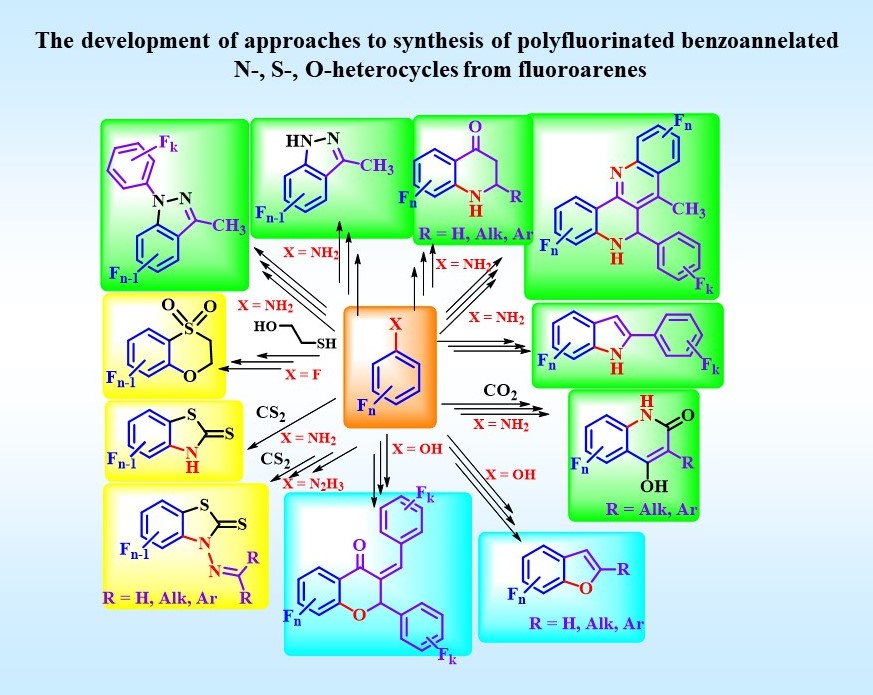
Fluorinated Hetarenes (Our research is focused on preparation of fluorinated (hetero)cyclic compounds, investigating their reactivity and areas of practical application)
-
L. Politanskaya, E. Tretyakov. p-Toluenesulfonic Acid Induced Conversion of Fluorinated Trimethylsilylethynylanilines into Aminoacetophenones: Versatile Precursors for the Synthesis of Benzoazaheterocycles. Synthesis 2018; 50(03): 555-564 doi:10.1055/s-0036-1591504.
-
L. Politanskaya, Z. Duan, I. Bagryanskaya, I. Eltsov, E. Tretyakov, Chanjuan. Xi. Highly efficient synthesis of polyfluorinated 2-mercaptobenzothiazole derivatives. Journal of Fluorine Chemistry, 2018, V. 212, Pp 130-136 doi:10.1016/j.jfluchem.2018.06.001.
-
L. Politanskaya, I. Bagryanskaya, E. Tretyakov. Synthesis of polyfluorinated arylhydrazines, hydrazones and 3-methyl-1-aryl-1H-indazoles. Journal of Fluorine Chemistry, 2018, V. 214, Pp 48-57 doi:10.1016/j.jfluchem.2018.06.011.
-
L. Politanskaya, N. Troshkova, E. Tretyakov, Ch. Xic. Synthesis of polyfluorinated bezofurans. Journal of Fluorine Chemistry, Volume 227, November 2019, 109371 doi:10.1016/j.jfluchem.2019.109371
-
L. Politanskaya, E. Tretyakov. Directed synthesis of fluorine containing 2,3-dihydrobenzo[b][1,4]oxathiine derivatives from polyfluoroarenes. Journal of Fluorine Chemistry, 2020, 109592 doi:10.1016/j.jfluchem.2020.109592
-
-
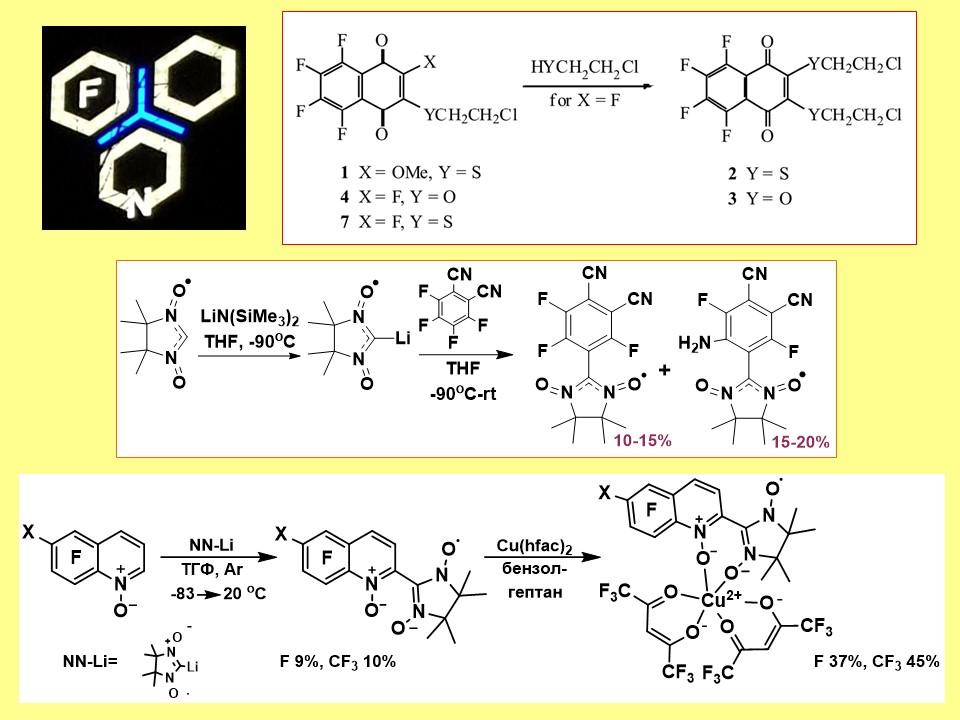
Application of aromatic nucleophilic substitution as a tool for developing new approaches to the construction and directed functionalization of polyfluorinated arenes (hetarenes) and quinones, including the introduction of radical groups.
-
E.V. Tretyakov, P.A. Fedyushin, E.V. Panteleeva, D.V. Stass, I.Yu. Bagryanskaya, I.V. Beregovaya, A.S. Bogomyakov. Substitution of a Fluorine Atom in Perfluorobenzonitrile by a Lithiated Nitronyl Nitroxide. J. Org. Chem., 2017, 82 (8), pp 4179-4185 doi:10.1021/acs.joc.7b00144.
-
A.D. Skolyapova, G.A. Selivanova, E.V. Tretyakov, T.F. Bogdanova, L.N. Shchegoleva, I.Yu. Bagryanskaya, L.Yu. Gurskaya, V.D. Shteingarts. Interaction of polyfluorinated 2-chloroquinolines with ammonia. Tetrahedron, 2017, V. 73, N 9, Pp 1219-1229 doi:10.1016/j.tet.2017.01.026.
-
P. Fedyushin, T. Rybalova, N. Asanbaeva, E. Bagryanskaya, A. Dmitriev, N. Gritsan, M. Kazantsev, E. Tretyakov. Synthesis of Nitroxide Diradical Using a New Approach. Molecules 2020, 25(11), 2701 doi:10.3390/molecules25112701.
-
P. Fedyushin, E. Panteleeva, I. Bagryanskaya, K. Maryunina, K. Inoue, D. Stass, E. Tretyakov. An approach to fluorinated phthalonitriles containing a nitronyl nitroxide or iminonitroxide moiety. Journal of Fluorine Chemistry, 2019, V. 217, Pp 1-7 doi:10.1016/j.jfluchem.2018.10.016
-
-
Functionally Oriented Synthesis of Organic and Hybrid Compounds (Our goals are to design and synthesize novel molecular structures in order to promote and control interesting chemical and physical properties)
-
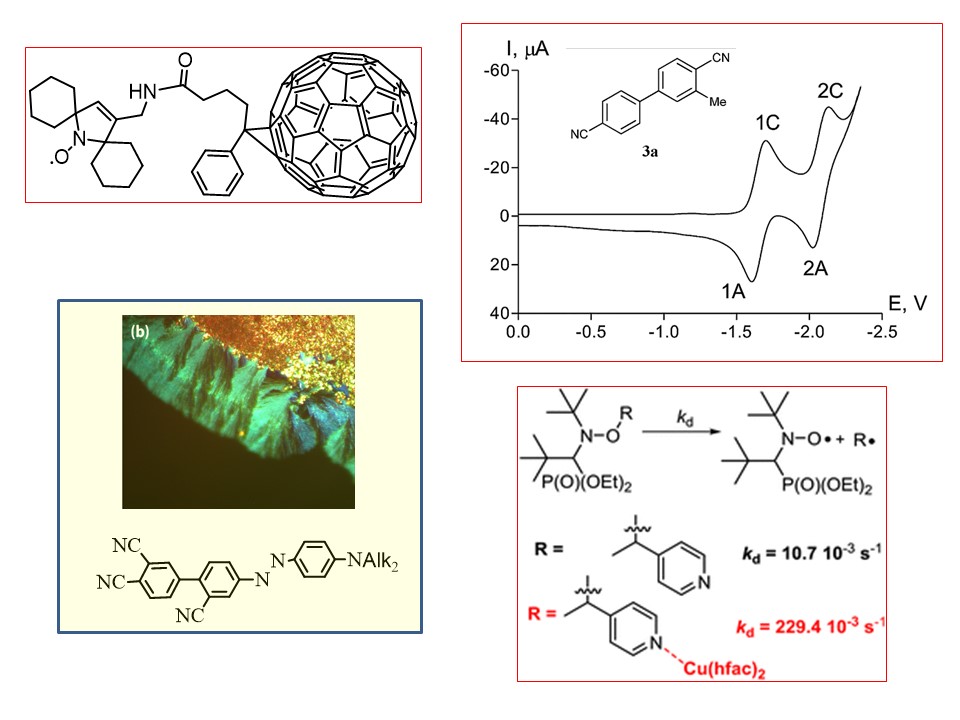
G. Audran, E.G. Bagryanskaya, P. Bremond, M.V. Edeleva, S.R.A. Marque, D.A. Parkhomenko, O.Yu. Rogozhnikova, V.M. Tormyshev, E.V. Tretyakov, D.V. Trukhin, S.I. Zhivetyeva. Trityl-based alkoxyamines as NMP controllers and spin-labels. Polym. Chem., 2016, 42(7), 6490-6499 doi:10.1039/C6PY01303A.
-
G. Audran, E. Bagryanskaya, I. Bagryanskaya, P. Bremond, M. Edeleva, S.R. Marque, D. Parkhomenko, E. Tretyakov, S. Zhivetyeva. C-ON bond homolysis of alkoxyamines triggered by paramagnetic copper(II) salts. Inorg. Chem. Front., 2016, 3, 1464-1472 doi:10.1039/C6QI00277C.
-
G.A. Selivanova, E.V. Tretyakov, E.V. Amosov, I.Yu. Bagryanskaya, V.G. Vasiliev, E.V. Vasilyev, V.D. Tikhova, E.V. Karpova, T.V. Basova, D.V. Stassd, V.D. Shteingarts. X-Ray induced phase transitions in 4-((4-(dibutylamino)phenyl)diazenyl)-biphenyl-2,3',4'-tricarbonitrile. Journal of Molecular Structure, V. 1107, 5 March 2016, Pp 242-248 doi:10.1016/j.molstruc.2015.11.060.
-
O. Guselnikova, S.R-A. Marque, E.V. Tretyakov, D. Mares, V. Jerabek, G. Audran, J-P. Joly, M. Trusova, V. Svorcik, O. Lyutakov, P. Postnikov. Unprecedented plasmon-induced nitroxide-mediated polymerization (PI-NMP): a method for preparation of functional surfaces. J. Mater. Chem. A, 2019, 2019, V. 7, N 2, Pp 12414-12419 , 2019 Journal of Materials Chemistry A HOT Papers doi:10.1039/C9TA01630A
-
-
Organic Conjugated Paramagnets and High Spin Molecules (Our research is in the area of Synthetic Radical Chemistry with emphasis on the design, synthesis and study of paramagnet molecules with the delocalized spin density)
-
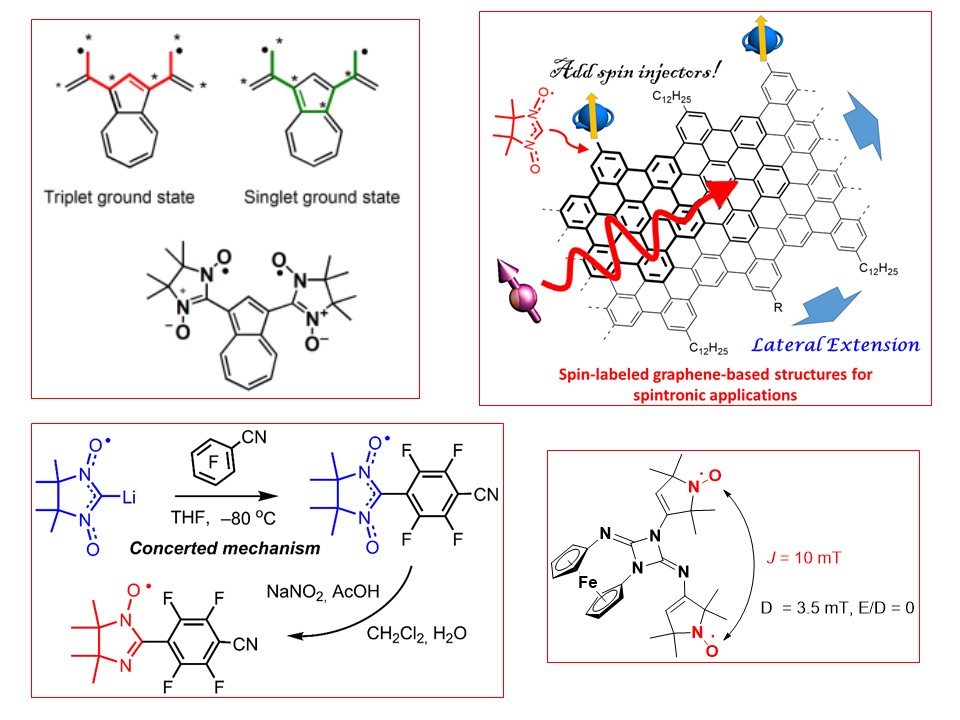
S.E. Tolstikov, E.V. Tretyakov, D.E. Gorbunov, I.F. Zhurko, M.V. Fedin, G.V. Romanenko, A.S. Bogomyakov, N.P. Gritsan, D.G. Mazhukin. Reaction of Paramagnetic Synthon, Lithiated 4,4,5,5-Tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl 3-oxide, with Cyclic Aldonitrones of the Imidazole Series. Chemistry - A European Journal, 2016, V. 22, N 41, Pp 14598-14604 doi:10.1002/chem.201602049.
-
V. Romanov, A. Vorob'ev, I. Bagryanskaya, D. Parkhomenko, E. Tretyakov. 1,3-Dipolar Cycloaddition of a Nitronyl Nitroxide-Substituted Alkyne to Heteroaromatic N-Imines. Australian Journal of Chemistry, 2017, 70(12) 1317-1320 doi:10.1071/CH17476.
-
K. Okada, M. Haraguchi, E. Tretyakov, N. Gritsan, G. Romanenko, D. Gorbunov, A. Bogomyakov, K. Maryunina, S. Suzuki, M. Kozaki, D. Shiomi, K. Sato, T. Takui, S. Nishihara, K. Inoue. (Azulene-1,3-diyl)-bis(nitronyl nitroxide) and -bis(iminonitroxide) and Their Copper Complexes. Chemistry - An Asian Journal, 2017, V. 12, N 22, Pp 2929-2941 doi:10.1002/asia.201701085.
-
L. Gurskaya, I. Bagryanskaya, E. Amosov, M. Kazantsev, L. Politanskaya, E. Zaytseva, E. Bagryanskaya, A. Chernonosov, E. Tretyakov. 1,3-Diaza[3]ferrocenophanes functionalized with a nitronyl nitroxide group. Tetrahedron, 2018, V. 74, N 15, Pp 1942-1950 doi:10.1016/j.tet.2018.02.062
-
M. Slota, A. Keerthi, W.K. Myers, E. Tretyakov, M. Baumgarten, A. Ardavan, H. Sadeghi, C.J. Lambert, A. Narita, K. Müllen, L. Bogani. Magnetic edge states and coherent manipulation of graphene nanoribbons. Nature, 2018, V. 557, N 7707, pp. 691-695 doi:10.1038/s41586-018-0154-7
-
-
- STAFF
Staff
- PUBLICATIONS
Publications over the past years
Laboratory staff publications (DB NIOCh)
Reviews, Articles
2022- CYANARYLATION OF FLUORINATED BENZONITRILES WITH TEREPHTHALONITRILE DIANION/ Panteleeva E.V., Peshkov R.Yu.// Chemistry for Sustainable Development. 2022. Т. 30. № 6. С. 617-624. doi:10.15372/CSD2022425
- L. Politanskaya, B. Khasano, A. Potapov
Synthetic approaches to fluorinated derivatives of 4-(vinylthio)pyridine
Journal of Fluorine Chemistry, V. 264, December 2022, 110063 doi:10.1016/j.jfluchem.2022.110063, IF=2.226 - L. Politanskaya, Ji.Wang, N.Troshkova, I.Chuikov, I. Bagryanskayaa
One-pot synthesis of fluorinated 2-arylchroman-4-one derivatives from 2-(triisopropylsilyl)ethynylphenols and aromatic aldehydes
Journal of Fluorine Chemistry V. 263, November 2022, 110045 doi:10.1016/j.jfluchem.2022.110045, IF=2.226 - N.B. Asanbaeva, L.Yu. Gurskaya, Yu.F. Polienko, T.V. Rybalova, M.S. Kazantsev, A.A. Dmitriev, N.P. Gritsan, N. Haro-Mares, T. Gutmann, G. Buntkowsky, E.V. Tretyakov, E.G. Bagryanskaya
Effects of Spiro-Cyclohexane Substitution of Nitroxyl Biradicals on Dynamic Nuclear Polarization
Molecules 2022, 27(10), 3252 doi:10.3390/molecules27103252, IF=4.927 - I.O. Timofeev, L.V. Politanskaya, E.V. Tretyakov, Yu.F. Polienko, V.M. Tormyshev, E.G. Bagryanskaya, O.A. Krumkacheva, M.V. Fedin
Fullerene-based triplet spin labels: methodology aspects for pulsed dipolar EPR spectroscopy
Phys. Chem. Chem. Phys., 2022, V.24, N 7, Pp. 4475-4484 doi:10.1039/D1CP05545C, IF=3.945 - L.Yu. Gurskaya, Yu.F. Polienko, T.V. Rybalova, N.P. Gritsan, A.A. Dmitriev, M.S. Kazantsev, E.V. Zaytseva, D.A. Parkhomenko, I.V. Beregovaya, G.A. Zakabluk, E.V. Tretyakov
Multispin Systems with a Rigid Ferrocene-1,1'-diyl-Substituted 1,3-Diazetidine-2,4-diimine Coupler: A General Approach
European journal of organic Chemistry, V. 2022, N 7, February 18, 2022, e202101234 doi:10.1002/ejoc.202101234, IF=3.261 - G.A. Selivanova, A.D. Skolyapova, J. Wang, E.V. Karpova, I. Shundrina, I.Yu. Bagryanskaya, E.V. Amosov
Azo dyes containing 1,3,4-thiadiazole fragment: synthesis, properties
New J. Chem., 2022, 46(4), 1929-1942 doi:10.1039/D1NJ05084B, IF=3.925
2021- A.O. Bryzgalov, T.A. Yakovleva, L.V. Politanskaya, I.Yu. Bagryanskaya, T.G. Tolstikova
Fluorinated 3,4-Dihydro-2H-1,4-Benzothiazin1,1-Dioxide Derivatives with Antiarrhythmic and Hypertensive Effects
American Journal of Biomedical Science & Research, 2021,13(4),328-338 doi:10.34297/AJBSR.2021.13.001882 - Azo chromophores for nonlinear-optical application/ G. A. Selivanova// Russian Chemical Bulletin, 2021, V. 70, N 2, Pp 213-238 doi:10.1007/s11172-021-3080-z, IF=1.222
- L. Politanskaya, E. Tretyakov, Chanjuan. Xi
Synthesis of polyfluorinated 4-hydroxyquinolin-2(1H)-ones based on the cyclization of 2-alkynylanilines with carbon dioxide
Journal of Fluorine Chemistry, 2021, V. 242, 109720 doi:10.1016/j.jfluchem.2020.109720, IF=2.05
2020- E.M. Kadilenko, N.P. Gritsan, E.V. Tretyakov, S.V. Fokin, G.V. Romanenko, A.S. Bogomyakov, D.E. Gorbunov, D. Schollmeyer, M. Baumgarten, V.I. Ovcharenko
A black-box approach to the construction of metal-radical multispin systems and analysis of their magnetic properties
Dalton Trans., 2020, 49(46), 16916-16927 doi:10.1039/D0DT03184D, IF=4.173 - L.V. Politanskaya, P.A. Fedyushin, T.V. Rybalova, A.S. Bogomyakov, N.B. Asanbaeva, E.V. Tretyakov
Fluorinated Organic Paramagnetic Building Blocks for Cross-Coupling Reactions
Molecules 2020, 25(22), 5427 doi:10.3390/molecules25225427, IF=3.266 - E.V. Tretyakov, S.I. Zhivetyeva, P.V. Petunin, D.E. Gorbunov, N.P. Gritsan,
I.Yu. Bagryanskaya, A.S. Bogomyakov, P.S. Postnikov, M.S. Kazantsev, M.E.
Trusova, I.K. Shundrina, E.V. Zaytseva, D.A. Parkhomenko, E.G. Bagryanskaya,
V. Ovcharenko
Ferromagnetically Coupled S = 1 Chains in Crystals of Verdazyl‐Nitronyl Nitroxide Diradicals
Angewandte Chemie International Edition, 2020, V. 59, N 46, Pp 20704-20710 doi:10.1002/anie.202010041, IF=12.959 - L. Politanskaya, I. Bagryanskaya, E. Tretyakov, Ch. Xic
Highly efficient synthesis of novel fluorinated 3-amino-2-mercaptobenzothiazole-2(3H)-thione derivatives
Journal of Fluorine Chemistry, 2020, V.239, 109628 doi:10.1016/j.jfluchem.2020.109628, IF=2.322 - V. Romanov, E. Tretyakov, G. Selivanova, J. Li, I. Bagryanskaya, A. Makarov, D. Luneau
Synthesis and Structure of Fluorinated (Benzo[d]imidazol-2-yl)methanols: Bench Compounds for Diverse Applications
Crystals 2020, 10(9), 786 doi:10.3390/cryst10090786, IF=2.404 - L. Gurskaya, T. Rybalova, I. Beregovaya, E. Zaytseva, M. Kazantsev, E. Tretyakov
Aromatic nucleophilic substitution: a case study of the interaction of a lithiated nitronyl nitroxide with polyfluorinated quinoline-N-oxides
Journal of Fluorine Chemistry, 2020, V. 237, 109613 doi:10.1016/j.jfluchem.2020.109613, IF=2.322 - I. Zayakin, I. Bagryanskaya, D. Stass, M. Kazantsev, E. Tretyakov
Synthesis and Structure of (Nitronyl Nitroxide-2-ido)(tert-butyldiphenylphosphine)gold(I) and -(Di(tert-butyl)phenylphosphine)gold(I) Derivatives; Their Comparative Study in the Cross-Coupling Reaction
Crystals 2020, 10(9), 770 doi:10.3390/cryst10090770, IF=2.404 - 2-diethylaminovinyl derivatives of halogenated 1,4-quinones: synthetic and structural aspects/ S. I. Zhivetyeva, I. A. Zayakin, I. Yu. Bagryanskaya & E. V. Tretyakov// Journal of Structural Chemistryб 2020, V. 61, N 8, Pp 1253-1259 doi:10.1134/S0022476620080107 , IF=0.745
- M.A. Gromova, Yu.V. Kharitonov, L.V. Politanskaya, E.V. Tretyakov, E.E. Shults
A facile approach to hybrid compounds containing a tricyclic diterpenoid and fluorine-substituted heterocycles
Journal of Fluorine Chemistry, 2020, V. 236, 109554 doi:10.1016/j.jfluchem.2020.109554, IF=2.332 - L. Politanskaya, E. Tretyakov
Directed synthesis of fluorine containing 2,3-dihydrobenzo[b][1,4]oxathiine derivatives from polyfluoroarenes
Journal of Fluorine Chemistry, 2020, 109592 doi:10.1016/j.jfluchem.2020.109592, IF=2.332 - From spin-labelled fused polyaromatic compounds to magnetically active graphene nanostructures/ Yu A Ten, N M Troshkova, E V Tretyakov// Russian Chemical reviews, 2020, V. 89, N 7, Pp 693-712 doi:10.1070/RCR4923?locatt=label:RUSSIAN, IF=4.75
- P. Fedyushin, T. Rybalova, N. Asanbaeva, E. Bagryanskaya, A. Dmitriev, N. Gritsan, M. Kazantsev, E. Tretyakov
Synthesis of Nitroxide Diradical Using a New Approach
Molecules 2020, 25(11), 2701 doi:10.3390/molecules25112701, IF=3.267 - Fluorinated benzimidazoles for medicinal chemistry and new materials/ Selivanova, G.A., Tretyakov, E.V.// Russ Chem Bull? 2020, V. 69, N 5, Pp 838-858 doi:10.1007/s11172-020-2842-3, IF=1.061
- D.E. Votkina, P.V. Petunin, S.I. Zhivetyeva, I.Yu. Bagryanskaya, M.N. Uvarov, M.S. Kazantsev, M.E. Trusova, E.V. Tretyakov, P.S. Postnikov
Preparation of Multi-spin Systems: a Case Study of Tolane-bridged Verdazyl-based Hetero-diradicals
European Journal of Organic Chemistry, 2020, V. 2020, N 13, Pp 1996-2004 doi:10.1002/ejoc.202000044, IF=2.889 - E. Tretyakov, A. Tkacheva, G. Romanenko, A. Bogomyakov, D. Stass, A. Maryasov, E. Zueva, B. Trofimov, V. Ovcharenko
(Pyrrole-2,5-Diyl)-Bis(Nitronyl Nitroxide) and-Bis(Iminonitroxide): Specific Features of the Synthesis, Structure, and Magnetic Properties
Molecules 2020, 25(7), 1503 doi:10.3390/molecules25071503, IF=3.267 - Method of preparation of alkylated 1,3-diphenylpropan-2-ones, the components for assembly of graphene nanostructures/ Yu. A. Ten, N. M. Troshkova, E. V. Tretyakov// Russian Chemical Bulletin, 2020, V. 69, Pp 172-175 doi:10.1007/s11172-020-2740-8, IF=1.061
- L. Politanskaya, E. Tretyakov, Ch. Xi
Synthesis of polyfluorinated o-hydroxyacetophenones - convenient precursors of 3-benzylidene-2-phenylchroman-4-ones
Journal of Fluorine Chemistry, V. 229, January 2020, 109435 doi:10.1016/j.jfluchem.2019.109435, IF=2.332
2019- E. Tretyakov, P. Fedyushin, E. Panteleeva, L. Gurskaya, T. Rybalova, A. Bogomyakov, E. Zaytseva, M. Kazantsev, I. Shundrina, V. Ovcharenko
Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides
Molecules 2019, 24(24), 4493 doi:10.3390/molecules24244493, IF=3.59 - L.Politanskaya, M.Petyuk,E.Tretyakov
Transformation of fluorinated 2-alkynylanilines by various catalytic systems
Journal of Fluorine Chemistry, 2019, V. 228, Art. Number 109394 doi:10.1016/j.jfluchem.2019.109394, IF=2.055 - L. Politanskaya, N. Troshkova, E. Tretyakov, Ch. Xi
Synthesis of polyfluorinated bezofurans
Journal of Fluorine Chemistry, Volume 227, November 2019, 109371 doi:10.1016/j.jfluchem.2019.109371, IF=2.055 - O. Zakharova, G. Nevinsky, L. Politanskaya, D. Baev, L. Ovchinnikova, E. Tretyakov
Evaluation of antioxidant activity and cytotoxicity of polyfluorinated diarylacetylenes and indoles toward human cancer cells
Journal of Fluorine Chemistry, V. 226, October 2019, 109353 doi:10.1016/j.jfluchem.2019.109353, IF=2.055 - O.A. Krumkacheva, I.O. Timofeev, L.V. Politanskaya, Yu.F. Polienko, E.V. Tretyakov, O.Yu. Rogozhnikova, D.V. Trukhin, V.M. Tormyshev, A.S. Chubarov, E.G. Bagryanskaya, M.V. Fedin
Triplet Fullerenes as Prospective Spin Labels for Nanoscale Distance Measurements by Pulsed Dipolar EPR
Angewandte Chemie International Edition, 2019, V. 58, N 38, Pp 13271-13275 doi:10.1002/anie.201904152, IF=12.256 - G. Audran, E. Bagryanskaya, I. Bagryanskaya, M. Edeleva, J.-P. Joly, S-R.A. Marque, A. Iurchenkova, P. Kaletina, S. Cherkasov, T.To. Hai, E. Tretyakov, S. Zhivetyeva
How intramolecular coordination bonding (ICB) controls the homolysis of the C-ON bond in alkoxyamines
RSC Adv., 2019,V. 9, N 44, Pp 25776-25789 doi:10.1039/C9RA05334D, IF=3.049 - O. Guselnikova, S.R-A. Marque, E.V. Tretyakov, D. Mares, V. Jerabek, G. Audran, J-P. Joly, M. Trusova, V. Svorcik, O. Lyutakov, P. Postnikov
Unprecedented plasmon-induced nitroxide-mediated polymerization (PI-NMP): a method for preparation of functional surfaces
J. Mater. Chem. A, 2019, 2019, V. 7, N 2, Pp 12414-12419 , 2019 Journal of Materials Chemistry A HOT Papers doi:10.1039/C9TA01630A, IF=10.733 - D. Stass, E. Tretyakov
Estimation of Absolute Spin Counts in Nitronyl Nitroxide-Bearing Graphene Nanoribbons
Magnetochemistry, 2019, 5(2), 32 (This article belongs to the Special Issue Controlling Molecular Nanomagnets) doi:10.3390/magnetochemistry5020032 - Organofluorine chemistry: promising growth areas and challenges/ Politanskaya L.V., Selivanova G.A., Panteleeva E.V., Tretyakov E.V., Platonov V.E., Nikulshin P.V., Vinogradov A.S., Zonov Ya.V., Karpov V.M., Mezhenkova T.V., Vasilyev A.V., Koldobskii A.B., Shilova O.S., Morozova S.M., Burgart Ya.V., Shchegolkov E.V., Saloutin V.I., Sokolov V.B., Aksinenko A.Yu., Nenajdenko V.G., Moskalik M.Yu., Astakhova V.V., Shainyan B.A., Tabolin A.A., Ioffe S.L., Muzalevskiy V.M., Balenkova E.S., Shastin A.V., Tyutyunov A.A., Boiko V.E., Igumnov S.M., Dilman A.D., Adonin N.Yu., Bardin V.V., Masoud S.M., Vorobyeva D.V., Osipov S.N., Nosova E.V., Lipunova G.N., Charushin V.N., Prima D.O., Makarov A.G., Zibarev A.V., Trofimov B.A., Sobenina L.N., Belyaeva K.V., Sosnovskikh V.Ya., Obydennov D.L., Usachev S.A.// RUSSIAN CHEMICAL REVIEWS, 2019, V. 55, N 5, Pp, IF=4.612
- V. Romanov, I. Bagryanskaya, N. Gritsan, D. Gorbunov, Yu. Vlasenko, M. Yusubov, E. Zaytseva, D. Luneau, E. Tretyakov
Assembly of Imidazolyl-Substituted Nitronyl Nitroxides into Ferromagnetically Coupled Chains
Crystals 2019, 9(4), 219 doi:10.3390/cryst9040219, IF=2.061 - Exploration of SNF-Approach toward Functionalized Nitronyl Nitroxides/ P. Fedyushin, L. Gurskaya, E. Panteleeva, B. Koshcheev, A. Maksimov, T.V. Rybalova, E. Zaytseva, E. Tretyakov// doi:10.17677/fn20714807.2019.02.04
- V. Morozov, E. Tretyakov
Spin polarization in graphene nanoribbons functionalized with nitroxide
Journal of Molecular Modeling, 2019, V.25, N 3, Article 58 doi:10.1007/s00894-019-3944-4, IF=1.335 - D.O. Prima, A.G. Makarov, I.Yu. Bagryanskaya, A.E. Kolesnikov, L.V. Zargarova, D.S. Baev, T.F. Eliseeva, L.V. Politanskaya, A.Yu. Makarov, Yu.G. Slizhov, A. V. Zibarev
Fluorine-Containing n-6 and Angulaand Linear n-6-n' (n, n' = 5, 6, 7) Diaza-Heterocyclic Scaffolds Assembled on Benzene Core in Unified Way
ChemistrySelect, 2019, V. 4, N 8, Pp 2383-2386 doi:10.1002/slct.201803970, IF=1.716 - R.Yu. Peshkov, Ch. Wang, E.V. Panteleeva, T.V. Rybalova, E.V. Tretyakov
Radical Anions of Aromatic Carbonitriles as Reagents for Arylation of Fluorinated Benzonitriles
J. Org. Chem., 2019, 84 (2), pp 963-972 doi:10.1021/acs.joc.8b02904, IF=4.745 - P. Fedyushin, E. Panteleeva, I. Bagryanskaya, K. Maryunina, K. Inoue, D. Stass, E. Tretyakov
An approach to fluorinated phthalonitriles containing a nitronyl nitroxide or iminonitroxide moiety
Journal of Fluorine Chemistry, 2019, V. 217, Pp 1-7 doi:10.1016/j.jfluchem.2018.10.016, IF=2.055
2018- D. Stass, E. Tretyakov
Estimation of Absolute Spin Counts in Nitronyl Nitroxide-Bearing Graphene Nanoribbons
Magnetochemistry, 2019, V.5, N 2, P 32-40 doi:10.3390/magnetochemistry5020032 - R.Yu. Peshkov, Ch. Wang, E.V. Panteleeva, E.V. Tretyakov
Synthesis of 4'-alkyl-[1,1'-biphenyl]-2,3'-dicarbonitriles via dimerisation of phthalonitrile radical anion in liquid ammonia (18-10764UP)
Arkivoc,2018, part vii, Pp 349-356 doi:10.24820/ark.5550190.p010.764, IF=1.47 - G.A. Selivanova, A.D. Skolyapova, R.I. Dralyuk, E.V. Karpova, I.K. Shundrina, I.Yu. Bagryanskaya, E.V. Amosov, T.V. Basova, E.V. Tretyakov
Solid-phase transitions of polymorphs of 4-(4-N,N-dialkylaminophenyl)azobiphenyl-2,3'4'-tricarbonitriles and their analogues
Thermochimica Acta, 2018, V. 669, Pp 88-98 doi:10.1016/j.tca.2018.08.022, IF=2.188 - Yu.A. Ten, O.G. Salnikov, S.A. Amitina, D.V. Stass, T.V. Rybalova, M.S. Kazantsev, A.S. Bogomyakov, E.A. Mostovich, D.G. Mazhukin
The Suzuki–Miyaura reaction as a tool for modification of phenoxyl-nitroxyl radicals of the 4H-imidazole N-oxide series
RSC Adv., 2018, V. 8, N 46, Pp 26099-26107 doi:10.1039/C8RA05103H, IF=2.936 - L. Politanskaya, I. Bagryanskaya, E. Tretyakov
Synthesis of polyfluorinated arylhydrazines, hydrazones and 3-methyl-1-aryl-1H-indazoles
Journal of Fluorine Chemistry, 2018, V. 214, Pp 48-57 doi:10.1016/j.jfluchem.2018.06.011, IF=1.879 - V.E. Romanov, I.Yu. Bagryanskaya, D.E. Gorbunov, N.P. Gritsan, E.V. Zaytseva, D. Luneau, E.V. Tretyakov
A Crystallographic Study of a Novel Tetrazolyl-Substituted Nitronyl Nitroxide Radical
Crystals 2018, 8(9), 334 doi:10.3390/cryst8090334, IF=2.144 - L. Politanskaya, Z. Duan, I. Bagryanskaya, I. Eltsov, E. Tretyakov, Chanjuan. Xi
Highly efficient synthesis of polyfluorinated 2-mercaptobenzothiazole derivatives
Journal of Fluorine Chemistry, 2018, V. 212, Pp 130-136 doi:10.1016/j.jfluchem.2018.06.001, IF=1.879 - S.I. Zhivetyeva, I.A. Zayakin, I.Yu. Bagryanskaya, E.V. Zaytseva, E.G. Bargyanskaya, E.V. Tretyakov
Interaction of a lithiated nitronyl nitroxide with polyfluorinated 1,4-naphthoquinones
Tetrahedron, 2018, V. 74, N 28, Pp 3924-3930 doi:10.1016/j.tet.2018.05.075, IF=2.377 - L. Politanskaya, T. Rybalova, O. Zakharova, G. Nevinsky, E. Tretyakov
p-Toluenesulfonic acid mediated one-pot cascade synthesis and cytotoxicity evaluation of polyfluorinated 2-aryl-2,3-dihydroquinolin-4-ones and their derivatives
Journal of Fluorine Chemistry, 2018, V.211, Pp 129-140 doi:10.1016/j.jfluchem.2018.04.005, IF=1.879 - A.D. Skolyapova, G.A. Selivanova, E.V. Tretyakov, I.Yu. Bagryanskaya, V.D. Shteingarts
Synthesis of polyfluorinated aminoquinolines via nitroquinolines
Journal of Fluorine Chemistry, 2018, V. 211, Pp 14-23 doi:10.1016/j.jfluchem.2018.04.006, IF=1.879 - M. Slota, A. Keerthi, W.K. Myers, E. Tretyakov, M. Baumgarten, A. Ardavan, H. Sadeghi, C.J. Lambert, A. Narita, K. Müllen, L. Bogani
Magnetic edge states and coherent manipulation of graphene nanoribbons
Nature, 2018, V. 557, N 7707, pp. 691-695 doi:10.1038/s41586-018-0154-7, IF=41.577 - G. Audran, E. Bagryanskaya, M. Edeleva, S. Marque, D. Parkhomenko, E. Tretyakov, S. Zhivetyeva
Coordination-Initiated Nitroxide-Mediated Polymerization (CI-NMP)
Australian Journal of Chemistry, 2018, V. 71, N 5, Pp 334-340 doi:10.1071/CH17570, IF=1.059 - A. Vorontsov, E. Tretyakov
Determination of Graphene Edges Energy using Hexagonal Graphene Quantum Dots and PM7 Method
Phys. Chem. Chem. Phys., 2018, V. 20, N 21, Pp. 14740-14752 doi:10.1039/C7CP08411K, IF=3.906 - G. Audran, E.G. Bagryanskaya, I.Yu. Bagryanskaya, M. Edeleva, P. Kaletina, S.R-A. Marque, D. Parkhomenko, E.V. Tretyakov, S.I. Zhivetyeva
The effect of the oxophilic Tb(III) cation on Csingle bond-ON bond homolysis in alkoxyamines
Inorganic Chemistry Communications, 2018, V. 91, Pp 5-7 doi:10.1016/j.inoche.2018.02.019, IF=1.81 - M.M. Petrova, S.I. Zhivetyeva, E.V. Tretyakov
Triplet Diradicals Derived From Quinone-Nitroxides: A Quantum-Chemical Study
SDRP Journal of Computational Chemistry & Molecular Modelling, 2018, V: 2, N 2 doi:10.25177/JCCMM.2.2.5 - L. Gurskaya, I. Bagryanskaya, E. Amosov, M. Kazantsev, L. Politanskaya, E. Zaytseva, E. Bagryanskaya, A. Chernonosov, E. Tretyakov
1,3-Diaza[3]ferrocenophanes functionalized with a nitronyl nitroxide group
Tetrahedron, 2018, V. 74, N 15, Pp 1942-1950 doi:10.1016/j.tet.2018.02.062, IF=2.377 - Molecular and Crystal Structure of 2-Amino-Polyfluorophenyl-4,4,5,5-Tetramethyl-4,5-Dihydro-1H-Imidazol- 3-Oxide-1-Oxyls/ E.V. Tretyakov, T.V. Makhneva, L.V. Politanskaya, I.Yu. Bagryanskaya, D.V. Stass// Journal of Structural Chemistry, 2018, V. 59, N 3, pp 689-696 doi:10.1134/S0022476618030289, IF=0.521
- P.A. Fedyushin, R.Yu. Peshkov, E.V. Panteleeva, E.V. Tretyakov, I.V. Beregovaya, Yu.V. Gatilov, V.D. Shteingarts
Purposeful regioselectivity control of the Birch reductive alkylation of biphenyl-4-carbonitrile
Tetrahedron, 2018, V. 74, N 8, Pages 842-851 doi:10.1016/j.tet.2017.12.046, IF=2.377 - S.I. Zhivetyeva, E.V. Tretyakov, I.Yu. Bagryanskaya
Synthesis of novel phosphonium betaines and bis-betaines derived from hexafluoro-1,4-naphthoquinone
Journal of Fluorine Chemistry, V. 206, February 2018, Pp. 19-28 doi:10.1016/j.jfluchem.2017.11.010, IF=1.879 - L. Politanskaya, E. Tretyakov
p-Toluenesulfonic Acid Induced Conversion of Fluorinated Trimethylsilylethynylanilines into Aminoacetophenones: Versatile Precursors for the Synthesis of Benzoazaheterocycles
Synthesis 2018; 50(03): 555-564 doi:10.1055/s-0036-1591504, IF=2.722
2017- V. Romanov, A. Vorob'ev, I. Bagryanskaya, D. Parkhomenko, E. Tretyakov
1,3-Dipolar Cycloaddition of a Nitronyl Nitroxide-Substituted Alkyne to Heteroaromatic N-Imines
Australian Journal of Chemistry, 2017, 70(12) 1317-1320 doi:10.1071/CH17476, IF=1.327 - A.S. Kondratyev, V.D. Shteingarts, V.V. Litvak, E.V. Tretyakov, A.V. Tkachev
Domino reaction of (2-haloethyl)polyfluorophenyl sulfides, sulfoxides, and sulfones with ammonia or amines: one-pot synthesis of 3,4-dihydro-2H-1,4-benzothiazines polyfluorinated at the benzene ring and the corresponding 1-oxides and 1,1-dioxides
Chemistry of Heterocyclic Compounds, 2017, V. 53, N 12, P 1350-1361 doi:10.1007/s10593-018-2217-y, IF=0.893 - K. Okada, M. Haraguchi, E. Tretyakov, N. Gritsan, G. Romanenko, D. Gorbunov, A. Bogomyakov, K. Maryunina, S. Suzuki, M. Kozaki, D. Shiomi, K. Sato, T. Takui, S. Nishihara, K. Inoue
(Azulene-1,3-diyl)-bis(nitronyl nitroxide) and -bis(iminonitroxide) and Their Copper Complexes
Chemistry - An Asian Journal, 2017, V. 12, N 22, Pp 2929-2941 doi:10.1002/asia.201701085, IF=4.83 - E. Tretyakov, A. Keerthi, M. Baumgarten, S. Veber, M. Fedin, D. Gorbunov, I. Shundrina, N. Gritsan
The Design of Radical Stacks: Nitronyl-Nitroxide-Substituted Heteropentacenes
ChemistryOpen, 2017, V. 6, N 5, Pp 642-652 doi:10.1002/open.201700110, IF=2.917 - V. Shelkovnikov, G. Selivanova, G. Lyubas, S. Korotaev, I. Shundrina, E. Tretyakov, E. Zueva, A. Plekhanov, S. Mikerin, A. Simanchuk
Second-order nonlinear optical properties of composite material of an azo-chromophore with a tricyanodiphenyl acceptor in a poly(styrene-co-methyl methacrylate) matrix
Optical Materials, 2017, V. 69, Pp 67-72 doi:10.1016/j.optmat.2017.04.008, IF=2.237 - Comparative Study of Toxicity of Alkoxyamines In Vitro and In Vivo/ N.A. Popova, G.M. Sysoeva, V.P. Nikolin, V.I. Kaledin, E.V. Tretyakov, M.V. Edeeva, S.M. Balakhnin, E.L. Lushnikova, G. Audran, S. Mark// Bulletin of Experimental Biology and Medicine, November 2017, V. 164, N 1, pp 49-53 doi:10.1007/s10517-017-3924-6, IF=0.456
- L.V. Saloutina, E.V. Tretyakov, P.A. Slepukhin, V.I. Saloutin, O.N. Chupakhin
Synthesis of Cyclic Vicinal Trifluoromethylated Hydroxylamine and Nitrone Based on Perfluorodiacetyl
Journal of Heterocyclic Chemistry, 2017, V. 54, N 3, Pp 1887-1890 doi:10.1002/jhet.2782, IF=0.893 - G. Audran, E. Bagryanskaya, I. Bagryanskaya, M. Edeleva, S.R.A. Marque, D. Parkhomenko, E. Tretyakov, S. Zhivetyeva
Zinc(II) Hexafluoroacetylacetonate Complexes of Alkoxyamines: NMR and Kinetic Investigations. First Step for a New Way to Prepare Hybrid Materials
ChemistrySelect, 2017, V. 2, N 12, Pp 3584-3593 doi:10.1002/slct.201700678 - E.V. Tretyakov, P.A. Fedyushin, E.V. Panteleeva, D.V. Stass, I.Yu. Bagryanskaya, I.V. Beregovaya, A.S. Bogomyakov
Substitution of a Fluorine Atom in Perfluorobenzonitrile by a Lithiated Nitronyl Nitroxide
J. Org. Chem., 2017, 82 (8), pp 4179-4185 doi:10.1021/acs.joc.7b00144, IF=4.848 - A.D. Skolyapova, G.A. Selivanova, E.V. Tretyakov, T.F. Bogdanova, L.N. Shchegoleva, I.Yu. Bagryanskaya, L.Yu. Gurskaya, V.D. Shteingarts
Interaction of polyfluorinated 2-chloroquinolines with ammonia
Tetrahedron, 2017, V. 73, N 9, Pp 1219-1229 doi:10.1016/j.tet.2017.01.026, IF=2.651 - Synthesis and study of CuII complex with nitroxide, a jumping crystal analog/ R. Z. Sagdeev, S. E. Tolstikov, S. V. Fokin, I. V. Obsharova, S. V. Tumanov, S. L. Veber, G. V. Romanenko, A. S. Bogomyakov, M. V. Fedin, E. V. Tretyakov, M. Halcrow, V. I. Ovcharenko// Russian Chemical Bulletin, February 2017, V. 66, N 2, pp 222-230 doi:10.1007/s11172-017-1722-y, IF=0.528
- I. Bagryanskaya, M. Fedin, D. Gorbunov, N. Gritsan, L. Gurskaya, M. Kazantsev, Yu. Polienko, D. Stass, E. Tretyakov
A Nitroxide Diradical Containing a Ferrocen-1,1'-diyl-substituted 1,3-Diazetidine-2,4-diimine Coupler
Tetrahedron Letters, 2017, V. 58, N 5, Pp 478-481 doi:10.1016/j.tetlet.2016.12.068, IF=2.193
2016- S.I. Zhivetyeva, O.D. Zakharova, L.P. Ovchinnikova, D.S. Baev, I.Yu. Bagryanskaya, V.D. Shteingarts, T.G. Tolstikova, G.A. Nevinsky, E.V. Tretyakov
Phosphonium betaines derived from hexafluoro-1,4-naphthoquinone: Synthesis and cytotoxic and antioxidant activities
Journal of Fluorine Chemistry, V. 192, Part A, December 2016, Pp 68-77 doi:10.1016/j.jfluchem.2016.10.014, IF=2.213 - E. Tretyakov, K. Okada, S. Suzuki, M. Baumgarten, G. Romanenko, A. Bogomyakov, V. Ovcharenko
Synthesis, structure and properties of nitronyl nitroxide diradicals with fused-thiophene couplers
JOURNAL OF PHYSICAL ORGANIC CHEMISTRY. V. 29, N 12, December 2016, Pp.725-734 doi:10.1002/poc.3561, IF=1.514 - G. Audran, E.G. Bagryanskaya, P. Bremond, M.V. Edeleva, S.R.A. Marque, D.A. Parkhomenko, O.Yu. Rogozhnikova, V.M. Tormyshev, E.V. Tretyakov, D.V. Trukhin, S.I. Zhivetyeva
Trityl-based alkoxyamines as NMP controllers and spin-labels
Polym. Chem., 2016, 42(7), 6490-6499 doi:10.1039/C6PY01303A, IF=5.687 - G. Audran, E. Bagryanskaya, I. Bagryanskaya, P. Bremond, M. Edeleva, S.R. Marque, D. Parkhomenko, E. Tretyakov, S. Zhivetyeva
C-ON bond homolysis of alkoxyamines triggered by paramagnetic copper(II) salts
Inorg. Chem. Front., 2016,3, 1464-1472 doi:10.1039/C6QI00277C, IF=4.532 - S.E. Tolstikov, E.V. Tretyakov, D.E. Gorbunov, I.F. Zhurko, M.V. Fedin, G.V. Romanenko, A.S. Bogomyakov, N.P. Gritsan, D.G. Mazhukin
Reaction of Paramagnetic Synthon, Lithiated 4,4,5,5-Tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl 3-oxide, with Cyclic Aldonitrones of the Imidazole Series
Chemistry - A European Journal, 2016, V. 22, N 41, Pp 14598-14604 doi:10.1002/chem.201602049, IF=5.77 - Synthesis of 4-(ω-X-alkyl)benzonitriles (X=1,3-dioxan-2-yl, CN, (CO2Et) by the reaction of terephthalonitrile dianion with ω-X-alkyl bromides in liquid ammonia/ R. Yu. Peshkov, Chynyan Wang, E. V. Panteleeva, E. V. Tretyakov, V. D. Shteingarts// Russian Chemical Bulletin, 2016, V. 65, N 10, pp 2430-2436 doi:10.1007/s11172-016-1602-x, IF=0.579
- L.V. Politanskaya, V.D. Shteingarts, E.V. Tretyakov
General and efficient synthesis of polyfluorinated 2-aminotolans and 2-arylindoles
Journal of Fluorine Chemistry, Journal of Fluorine Chemistry, 2016, V. 188, August 2016, Pp 85-98 doi:10.1016/j.jfluchem.2016.06.010, IF=2.213 - R.Yu. Peshkov, E.V. Panteleeva, W. Chunyan, E.V. Tretyakov, V.D. Shteingarts
One-pot synthesis of 4'-alkyl-4-cyanobiaryls on the basis of the terephthalonitrile dianion and neutral aromatic nitrile cross-coupling
Beilstein Journal of Organic Chemistry, 2016, V.12, Pp 1577-1584 doi:10.3762/bjoc.12.153, IF=2.697 - E.V. Tretyakov, R.Yu. Peshkov, E.V. Panteleeva, A.S. Scrypnik, D.V. Stass, G.V. Romanenko, V.I. Ovcharenko
Addition of Cyanomethyl Anion to the Cyano Group of 2-Cyano-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-3-oxide-1-oxyl, a Nitronyl Nitroxide
Tetrahedron Letters, V. 57, N 21, 25 May 2016, Pp 2327-2330 doi:10.1016/j.tetlet.2016.04.070, IF=2.346 - L.Yu. Gurskaya, D.S. Belyanskaya, D.S. Ryabukhin, D.I. Nilov, I.A. Boyarskaya, A.V. Vasilyev
Reactions of N,3-diarylpropiolamides with arenes under superelectrophilic activation: synthesis of 4,4-diaryl-3,4-dihydroquinolin-2(1H)-ones and their derivatives
Beilstein J. Org. Chem. 2016, 12, 950-956. doi:10.3762/bjoc.12.93, IF=2.697 - I.Yu. Bagryanskaya, L.V. Politanskaya, E.V. Tretyakov
Frequently used, but still unknown: Terbium(III) tris-hexafluoroacetylacetonate dihydrate
Inorganic Chemistry Communications, April 2016, V. 66, Pp 47-50 doi:10.1016/j.inoche.2016.02.009, IF=1.761 - G.A. Selivanova, E.V. Tretyakov, E.V. Amosov, I.Yu. Bagryanskaya, V.G. Vasiliev, E.V. Vasilyev, V.D. Tikhova, E.V. Karpova, T.V. Basova, D.V. Stass, V.D. Shteingarts
X-Ray induced phase transitions in 4-((4-(dibutylamino)phenyl)diazenyl)-biphenyl-2,3',4'-tricarbonitrile
Journal of Molecular Structure, V. 1107, 5 March 2016, Pp 242-248 doi:10.1016/j.molstruc.2015.11.060, IF=1.779 - O.D. Zakharova, L.P. Ovchinnikova, S.I. Zhivetyeva, L.I. Goryunov, V.D. Shteingarts, E.V. Tretyakov, G.A. Nevinsky
Synthesis and Evaluation of Cytotoxicity and Antioxidant Properties of Polyfluorinated Phosphorus-containing 1,4-Benzoquinones and 1,4-Naphthoquinones
Adv. Res., 2016, 6(6), 1-12 doi:10.9734/AIR/2016/24265 - Microwave-Assisted Synthesis of Phthalocyanine Zinc Complexes Derived from Aminotricyanobiphenyl-Based Azo Dyes/ G.A. Selivanova, E. V. Amosov, V. G. Vasilyev, E. A. Lukyanets, E. V. Tretyakov, V.D. Shteingarts// Macroheterocycles 2016 9(1) 80-88 doi:10.6060/mhc151192s, IF=0.804
2015- S.I. Zhivetyeva, G.A. Selivanova, L.I. Goryunov, I.Yu. Bagryanskaya, V.D. Shteingarts
Triphenylphosphanodefluorination of Fluoranil and its Derivatives
Journal of Fluorine Chemistry, V. 180, December 2015, Pp 21-32 doi:10.1016/j.jfluchem.2015.08.018, IF=1.947 - I. Yu. Barskaya, S.L. Veber, S.V. Fokin, E.V. Tretyakov, E.G. Bagryanskaya, V.I. Ovcharenko, M.V. Fedin
Structural specifics of light-induced metastable states in copper(II)-nitroxide molecular magnets
Dalton Trans., 2015,44, 20883-20888 doi:10.1039/C5DT03683F, IF=4.197 - L. V. Politanskaya, I. P. Chuikov, E. V. Tretyakov, V. D. Shteingarts, L. P. Ovchinnikova, O. D. Zakharova, G. A. Nevinsky
An effective two-step synthesis, fluorescent properties, antioxidant activity and cytotoxicity evaluation of benzene-fluorinated 2,2-dimethyl-2,3-dihydro-1H-quinolin-4-ones
Journal of Fluorine Chemistry, V. 178, October 2015, Pp 142-153 doi:10.1016/j.jfluchem.2015.07.006, IF=1.947 - L. Politanskaya, V. Shteingarts, E. Tretyakov, A. Potapov
The p-toluenesulfonic acid-catalyzed transformation of polyfluorinated 2-alkynylanilines to 2-aminoarylketones and indoles
Tetrahedron Letters, V. 56, N 39, 23 September 2015, Pp 5328-5332 doi:10.1016/j.tetlet.2015.07.078, IF=2.378 - V.I. Borovkov, I.V. Beregovaya, L.N. Shchegoleva, S.V. Blinkova, D.A. Ovchinnikov, L.Yu. Gurskaya, V.D. Shteingarts, V.A. Bagryansky, Yu.N. Molin
Structure and Stability of Pentafluoroaniline and 4-Aminononafluorobiphenyl Radical Anions: Optically Detected Electron Paramagnetic Resonance, Time-Resolved Fluorescence, Time-Resolved Magnetic Field Effect, and Quantum Chemical Study
J. Phys. Chem. A, 2015, 119 (31), pp 8443–8451 doi:10.1021/acs.jpca.5b02617, IF=2.693 - R.Yu. Peshkov, E.V. Panteleeva, L.N. Shchegoleva, I.Yu. Bagryanskaya, T.V. Rybalova, N.V. Vasilieva, V.D. Shteingarts
Synthesis of 2-X-, 3-X-4,4'-Dicyanobiphenyls (X = CH3, OCH3, F) by Cross-Coupling of the Terephthalonitrile Dianion with Substituted Benzonitriles
European Journal of Organic Chemistry, V. 2015, N 20, pp 4524-4531, July 2015 doi:10.1002/ejoc.201500295, IF=3.65 - O.D. Zakharova, L.P. Ovchinnikova, L.I. Goryunov, N.M. Troshkova, V.D. Shteingarts, G.A. Nevinsky
Antioxidant Activity and Cytotoxicity of Polyfluorinated 1, 4- Naphthoquinones with Alkylating Functions in the Quinone Moiety
International Journal of Medicine and Pharmaceutical Sciences (IJMPS), 2015, V.5, N 1, Pp. 11-20 (Paper ID=IJMPSFEB20151)
2014- S.I. Zhivetyeva, L.I. Goryunov, I.Yu. Bagryanskaya, J. Grobe, V.D. Shteingarts, E.U. Wurthwein
Phosphinodefluorination of polyfluorobenzenes by silylphosphines Ph(R)PSiMe3 (R = Me, Ph): Further experimental and computational evidences for the concerted A(N)D(N) mechanism of aromatic nucleophilic substitution
Journal of Fluorine Chemistry, 2014, V. 164, Pp. 58-69. doi:10.1016/j.jfluchem.2014.04.012, IF=1.952 - N.M. Troshkova, L.I. Goryunov, V.D. Shteingarts, O.D. Zakharova, L.P. Ovchinnikova, G.A. Nevinsky
Synthesis and cytotoxicity evaluation of polyfluorinated 1,4-naphthoquinones containing amino acid substituents
Journal of Fluorine Chemistry, 2014, V.164, Pp 18-26. doi:10.1016/j.jfluchem.2014.04.014, IF=1.952
2013- L.Yu. Gurskaya, V.D. Shteingarts
Ortho-hydrodefluorination of polyfluorinated 4-acetamidobiphenyls and synthesis of polyfluorinated 6-phenylquinolines
Journal of Fluorine Chemistry, 2013, V. 156, P. 214–219 doi:10.1016/j.jfluchem.2013.09.015, IF=1.939 - L. V. Politanskaya, I. P. Chuikov, V. D. Shteingarts
Synthesis of indoles with a polyfluorinated benzene ring
Tetrahedron, 2013, V. 69., N 39, P. 8477-8486. doi:10.1016/j.tet.2013.07.037, IF=2.802 - A.G. Potapov, L.V. Politanskaya
The study of the adsorption of 1,3-diethers on the MgCl2 surface
Journal of Molecular Catalysia A-Chemical, 2013, V. 368, P. 159-162. doi:10.1016/j.molcata.2012.12.004, IF=3.187 - Synthesis of phthalonitriles containing omega-alkenyl, omega-(alkylsulfanyl)alkyl, and omega-(alkylsulfonyl)alkyl substituents and phthalocyanine derivatives based thereon/ E. V. Panteleeva, A. S. Kondrat’ev, L. I. Goryunov, V. V. Koval’, E. A. Luk’yanets, V. D. Shteingarts// Russian Journal of Organic Chemistry, 2013, V.49, N 1, pp 138-144 doi:10.1134/S1070428013010235, IF=0.513
2012- Alkoxylation of 4-chloronitrobenzene with aliphatic alcohols and glycols in the presence of NaOH/ E.V. Malykhin, V.D. Shteingarts// RUSS J APPL CHEM+, 2012, V. 85, N 8, pp: 1232-123 doi:10.1134/S1070427212080162, IF=0.282
- G.A. Selivanova, A.V. Reshetov, I.Yu. Bagryanskaya, V.D. Shteingarts
Skraup-like cyclization of polyfluoro-2-naphthylamines: Vicarious electrophilic substitution of fluorine
J. Fluorine Chem., 2012, V. 137, 113–116. doi:10.1016/j.jfluchem.2012.03.001, IF=2.32 - G.A. Selivanova, A.V. Reshetov, I.V. Beregovaya, N.V. Vasil’eva, I.Yu. Bagryanskaya, V.D. Shteingarts
Hydrodefluorination of polyfluoro-2-naphthylamines by Zn in aqueous NH3: A correlation of the product distribution and the computationally predicted regioselectivity of the substrate radical anion fragmentation
J. Fluorine Chem., 2012, V. 137, 64–72. doi:10.1016/j.jfluchem.2012.02.012, IF=2.32 - L.Yu. Gurskaya, G.A. Selivanova, V.D. Shteingarts
Interaction of quinolines polyfluorinated on the benzene moiety with sodium and potassium amides in liquid ammonia
J. Fluorine Chem., 2012, V. 136, 32–37. doi:10.1016/j.jfluchem.2012.01.007, IF=2.32 - L.V. Politanskaya, I.P. Chuikov, Е.А. Kolodina, M.S. Shvartsberg, V.D. Shteingarts
Synthesis of polyfluorinated orthoalkynylanilines
J. Fluorine Chem., 2012, V. 135, 97-107. doi:0.1016/j.jfluchem.2011.09.008, IF=2.32
2011- A.V. Reshetov, G.A. Selivanova, L.V. Politanskaya, I.V. Beregovaya, L.N. Shchegoleva, N.V. Vasil’eva, I.Yu. Bagryanskaya, V.D. Shteingarts
Hydrodefluorination of N-acetylheptafluoro-2-naphthylamine by zinc in aqueous ammonia: synthetic outcomes and mechanistic considerations
ARKIVOC, 2011, (viii), 242–262.full text doi:10.3998/ark.5550190.0012.818, IF=1.95 - L.I. Goryunov, S.I. Zhivetyeva, G.A. Nevinsky, V.D. Shteingarts
Synthesis of diphenyl(X)phosphonium betaines (X = CH3, C6H5, 2,5-F2C6H3) from hexafluoro-1,4-naphthoquinone
ARKIVOC, 2011, (viii), 185-191.full text doi:10.3998/ark.5550190.0012.814, IF=1.95 - E.V. Panteleeva, V.D. Shteingarts, I.Yu. Bagryanskaya, G.E. Sal’nikov
The formation of dicyanoterphenyls by the interaction of terephthalonitrile dianion with biphenylcarbonitriles in liquid ammonia
ARKIVOC, 2011, (viii), 123-133.full text doi:10.3998/ark.5550190.0012.808, IF=1.95 - Synthesis of 2,3',4'-tricyanobiphenyl derivatives and tetraphenylphthalocyanines based thereon/ G.A. Selivanova, E.V. Amosov, L.I. Goryunov, S.V. Balina, V.G. Vasil'ev, G.E. Sal'nikov, E.A. Luk'yanets, V.D. Shteingarts// RUSS J ORG CHEM+, 2011, V. 47, N 8, pp. 1240-1246. doi:10.1134/S1070428011080215, IF=0.634
- Synthesis of (2-X,3-Y-Phenyl)dimethylphosphanes (X, Y = Me2P, H; Me2P, F; Br, F) and their complexes with PdCl2/ L.I. Goryunov, V.D. Shteingarts, J. Grobe, R. Mews// RUSS J ORG CHEM+, 2011, V. 47, N 5, pp. 780-782. doi:10.1134/S1070428011050204, IF=0.634
- O.D. Zakharova, L.P. Ovchinnikova, L.I. Goryunov, N.M. Troshkova, V.D. Shteingarts, G.A. Nevinsky
Cytotoxicity of new polyfluorinated 1,4-naphthoquinones with divers substituents in the quinine moiety
Bioorg. Med. Chem. 2011, V. 19, 256-260. doi: 10.1016/j.bmc.2010.11.027, IF=2.977
2010- Synthesis of Fluorine-Containing Poly(phenylamino)-1,4-naphthoquinones/ N.M. Troshkova, L.I. Goryunov, V.D. Shteingarts// RUSS J ORG CHEM+, 2010, V. 46, N 10, pp 1585-1587. doi:10.1134/S1070428010100271, IF=0.524
- O.D. Zakharova, L.P. Ovchinnikova, L.I. Goryunov, N.M. Troshkova, V.D. Shteingarts, G.A. Nevinsky
Cytotoxicity of new alkylamino- and phenylamino-containing polyfluorinated derivatives of 1,4-naphthoquinone
Eur. J. Med. Chem., 2010, 45, 2321–2326. doi:10.1016/j.ejmech.2010.02.009, IF=3.269 - O.A. Zakharova, L.I. Goryunov, N.M. Troshkova, L.P. Ovchinnikova, V.D. Shteingarts, G.A. Nevinsky
Cytotoxicity of new n-butylamino and sulfur-containing derivatives of polyfluorinated 1,4-naphthoquinone
Eur. J. Med. Chem., 2010, 45, 270-274. doi:10.1016/j.ejmech.2009.10.00, IF=3.269 - L.I. Goryunov, J. Grobe, D. Le Van, V.D. Shteingarts, R. Mews, E. Lork, E.-U. Wurthwein
Di- and Trifluorobenzenes in Reactions with Me2EM (E = P, N; M = SiMe3, SnMe3, Li) Reagents: Evidence for the Concerted Mechanism of Aromatic Nucleophilic Substitution
Eur. J. Org. Chem., 2010, 6, 1111–1123. doi:10.1002/ejoc.200900880, IF=3.95 - N.M. Troshkova, L.I. Goryunov, Yu.V. Gatilov, G.A. Nevinsky, V.D. Shteingarts
Aminodefluorination of 2-X-pentafluoro-1,4-naphthoquinones (X = NHnBu, NEt2, and OMe)
J. Fluorine Chem., 2010, V. 131, N 1, 70-77. doi:10.1016/j.jfluchem.2009.10.007, IF=1.73 - V.A. Loskutov, Y.V. Gatilov, V.D. Shteingarts
An unexpected skeletal transformation of 1,4-dihydroxythioxanthen-9-one on treatment with iodic acid: the first construction of the 2-(5-oxofuran-2-ylidene)-1-benzothienyl-3-one core
Tetrahedron Letters, 2010, V. 51, N 49, Pp. 6396–6398. doi:10.1016/j.tetlet.2010.09.100, IF=2.66
2009- Reaction of chloropentafluorobenzene and 2,3-and 2,6-difluorobromobenzenes with lithium dimethylphosphide/ L.I. Goryunov, J. Grobe, V.D. Shteingarts, R. Mews, E.U. Wurthwein// Russian Journal of Organic Chemistry, 2009, V. 45, N 12, pp 1859-1861 doi:10.1134/S1070428009120197, IF=0.556
- Synthesis of beta-Functionalized Ethyl Polyfluoroaryl Sulfides, Sulfoxides, and Sulfones Underlain by Pentafluorobenzoic Acid/ V.V. Litvak, A.S. Kondrat'ev, V.D. Shteimgarts// Russian Journal of Organic Chemistry, 2009, V. 45, N 11, pp 1637-1643. doi:10.1134/S1070428009110104, IF=0.556
- L.Yu. Safina, G.A. Selivanova, K.Yu. Koltunov, V.D. Shteingarts
Synthesis of polyfluorinated 4-phenyl-3,4-dihydroquinolin-2-ones and quinolin-2-ones via superacidic activation of N-(polyfluorophenyl)cinnamamide
Tetrahedron Lett., 2009, V. 50, N 37, pp 5245–5247. doi:10.1016/j.tetlet.2009.07.013, IF=2.538 - Synthesis of 2-aminopentafluoro-1,4-naphthoquinone derivatives/ L.I. Goryunov, N.M. Troshkova, G.A. Nevinskii, V.D. Shteingarts// Russian Journal of Organic Chemistry, 2009, V. 45, N 6, pp 835-841 doi:10.1134/S1070428009060050, IF=0.556
- Reaction of quinolines fluorinated at the benzene ring with nitrogen-centered nucleophiles/ L.Yu. Safina, G.A. Selivanova, I.Yu. Bagryanskaya, V.D. Shteingarts// Russian Chemical Bulletin, 2009, V. 58, N 5, pp 1049-1061 doi:10.1007/s11172-009-0134-z, IF=0.469
2008- L.I. Goryunov, J. Grobe, V.D. Shteingarts
Compounds R1R2EMMe3 (E = P, As; M = Si,Sn) - Convenient and Versatile Reagents for the Synthesis of Tertiary Fluoroarylphosphanes and -Arsanes
Coll. Czech. Chem. Commun., 2008, V. 73, N 12, 1612–1622. doi:10.1135/cccc20081612, IF=0.879 - X-ray and quantum-topological studies of intermolecular interactions in partially fluorinated quinoline crystals/ I. Yu. Bagryanskaya, M. A. Grishina, L. Yu. Safina, G. A. Selivanova, V. A. Potemkin, Yu. V. Gatilov// Journal of Structural Chemistry, 2008, V. 49, N 5, pp 901-908 doi:10.1007/s10947-008-0155-8, IF=0.481
- Anionic reduced forms of electron-deficient arenes in reactions with C - C bond formation/ T.A. Vaganova, E.V. Panteleeva, V.D. Shteingarts// Russian Chemical Reviews,2008., V. 77, N 7, Pp 601-619. doi:10.1070/RC2008v077n07ABEH003776, IF=1.893
- Reductive activation of arenecarbonitriles for the reactions with some carbon-centered electrophiles: The reaction mechanisms and synthetic applications/ T.A. Vaganova, E.V. Panteleeva, V.D. Shteingarts// Russian Chemical Bulletin, 2008, V. 57, N 4, Pp 768-779. doi:10.1007/s11172-008-0116-6, IF=0.537
- N.V. Vasilieva, I.G. Irtegova, T.A. Vaganova, V.D. Shteingarts
Electrochemical, ESR and quantum chemical study of 1-substituted naphthalenes and their radical anions
J. Phys. Org. Chem., 2008, V. 21, N 1, 73–78. doi:10.1002/poc.1288, IF=1.593
2007- Arene complexes of transition metals in reactions with nucleophilic reagents: XXX. Reaction of π-halomesitylene [Tetramethyl(ethyl)cyclopentadienyl]rhodium(II) complexes with anions derived from CH acids/ L. I. Goryunov, N. M. Romanova, G. S. Zhilovskii, V. D. Shteingarts// Russian Journal of Organic Chemistry, 2007, V. 43, N 12, pp 1765-1772 doi:10.1134/S1070428007120056, IF=0.492
- V.D. Shteingarts
Recent advances in practice and theory of polyfluoroarene hydrodehalogenation
J. Fluorine Chem., 2007, V. 128, N 7, 795-805. doi:10.1016/j.jfluchem.2007.02.019, IF=1.514 - Reductive activation of arenes - 22. Reactions of the terephthalonitrile radical anion and dianion with alpha,omega-dibromoalkanes. New evidence for the charge transfer complex as a key intermediate in the reactions of the dianion/ E. V. Panteleeva, M. Yu. Lukyanova, L. M. Pokrovsky, V. D. Shteingarts// Russian Chemical Bulletin, 2007, V. 56, N 6, pp 1110-1118 doi:10.1007/s11172-007-0168-z, IF=0.504
- Researcher, educator, and science administrator/ V. D. Shteingarts// Herald of the Russian Academy of Sciences, 2007, Volume 77, Issue 3, pp 262-269 doi:10.1134/S1019331607030094, IF=0.149
- E.V. Panteleeva, G. Haufe, V.D. Shteingarts
Short Access to para-Alkenylbenzonitriles. Reaction of Anionic Reduced Forms of Terephthalonitrile with Alkenyl Bromides
SynLett., 2007, N 10, 1616-1618. doi:10.1055/s-2007-982549, IF=2.838 - S.S. Laev, L.Yu. Gurskaya, G.A. Selivanova, I.V. Beregovay, L.N. Shchegoleva, N.V. Vasil'eva, M.M. Shakirov, V.D. Shteingarts
N-Acetylation as a means to activate polyfluoroarylamines for selectyive ortho-hydrodefluorination by zinc in aqueous ammonia: a concise route to polyfluorobenzo Azaheterocycles
Eur. J. Org. Chem., 2007, N 2, 306-316. doi:10.1002/ejoc.200600684, IF=2.769
2006- Reductive activation of arenes: XIX. Mechanism and some synthetic applications of the alkylation of phthalodinitrile radical anion/ E. V. Panteleeva, T. A. Vaganova, E. A. Luk'yanets, V. D. Shteingarts// Russian Journal of Organic Chemistry, 2006, V. 42, N 9, pp 1280-1288 doi:10.1134/S1070428006090053, IF=0.419
- Reductive activation of arenes/ T. A. Vaganova, E. V. Panteleeva, P. S. Yuferov, Yu. V. Rebitva, V. D. Shteingarts// Russian Chemical Bulletin, 2006, V. 55, N 6, pp 981-986 doi:10.1007/s11172-006-0366-0, IF=0.589
- Reductive activation of arenes/ T. A. Vaganova, P. S. Yuferov, L. I. Goryunov, E. V. Panteleeva, G. E. Sal'nikov, L. N. Shchegoleva, V. I. Mamatyuk, V. D. Shteingarts// Russian Chemical Bulletin, 2006, V. 55, N 6, pp 976-980 doi:10.1007/s11172-006-0365-1, IF=0.589