Информация о публикации, соавторами которой являются сотрудники Института к.б.н. Д.С. Баев (снс,ЛФИ), д.б.н., проф. Т.Г. Толстикова (завлаб ЛФИ), д.х.н.О.И. Яровая (внс, ЛФАВ) и чл.-корр. РАН, д.х.н., проф. Н.Ф. Салахутдинов (зав. отделом медицинской химии), размещена на обложке журнала ACS Medicinal Chemistry Letters, (IF 4,345)

About the Cover:
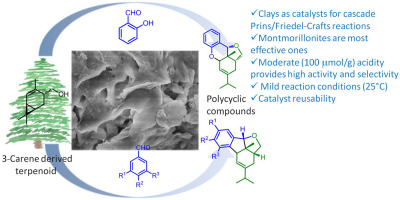
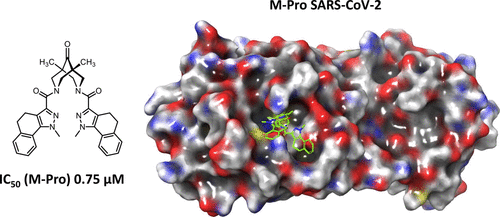
Results of the experiments performed with bispidine compounds showed that 14 compounds exhibited anti-3CLpro activity in the concentration range of 1–10 µM, and 3 samples exhibited submicromolar activity.
View the article.
Design and Evaluation of Bispidine-Based SARS-CoV-2 Main Protease Inhibitors
Dmitriy Shcherbakov, Dmitriy Baev, Mikhail Kalinin, Alexander Dalinger, Varvara Chirkova, Svetlana Belenkaya, Aleksei Khvostov, Dmitry Krut’ko, Aleksei Medved’ko, Ekaterina Volosnikova, Elena Sharlaeva, Daniil Shanshin, Tatyana Tolstikova, Olga Yarovaya*, Rinat Maksyutov, Nariman Salakhutdinov, and Sergey Vatsadze
ACS Med. Chem. Lett. 2022, 13, 1, 140–147
Publication Date (Web):September 29, 2021
doi:10.1021/acsmedchemlett.1c00299
Abstract
For the first time, derivatives of 3,7-diazabicyclo[3.3.1]nonane (bispidine) were proposed as potential inhibitors of the SARS-CoV-2 main viral protease (3-chymotrypsin-like, 3CLpro). Based on the created pharmacophore model of the active site of the protease, a group of compounds were modeled and tested for activity against 3CLpro. The 3CLpro activity was measured using the fluorogenic substrate Dabcyl-VNSTLQSGLRK(FAM)MA; the efficiency of the proposed approach was confirmed by comparison with literature data for ebselen and disulfiram. The results of the experiments performed with bispidine compounds showed that 14 compounds exhibited activity in the concentration range 1–10 μM, and 3 samples exhibited submicromolar activity. The structure–activity relationship studies showed that the molecules containing a carbonyl group in the ninth position of the bicycle exhibited the maximum activity. Based on the experimental and theoretical results obtained, further directions for the development of this topic were proposed.
Альметрики: