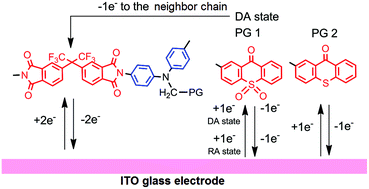
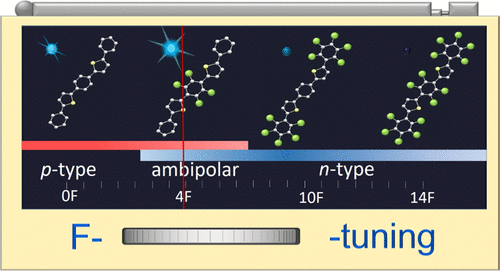
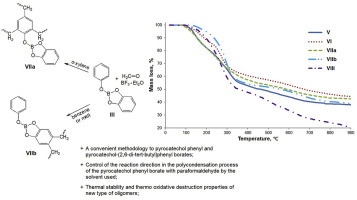
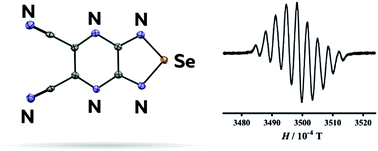
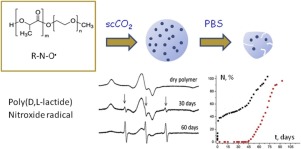
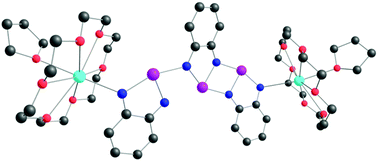
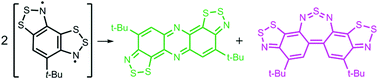
В журналу Bioorganic Chemistry (IF 3,926) опубликована статья с участием сотрудников Института к.х.н. С.О. Куранова (нс, ЛНТПС ), д.х.н. О.А. Лузиной (внс ЛФАВ) и д.х.н., чл.-корр. РАН, проф. Н.Ф. Салахутдинова (зав.оделом, ОМХ, завлаб , ЛФАВ):
Exploring bulky natural and natural-like periphery in the design of p-(benzyloxy)phenylpropionic acid agonists of free fatty acid receptor 1 (GPR40)
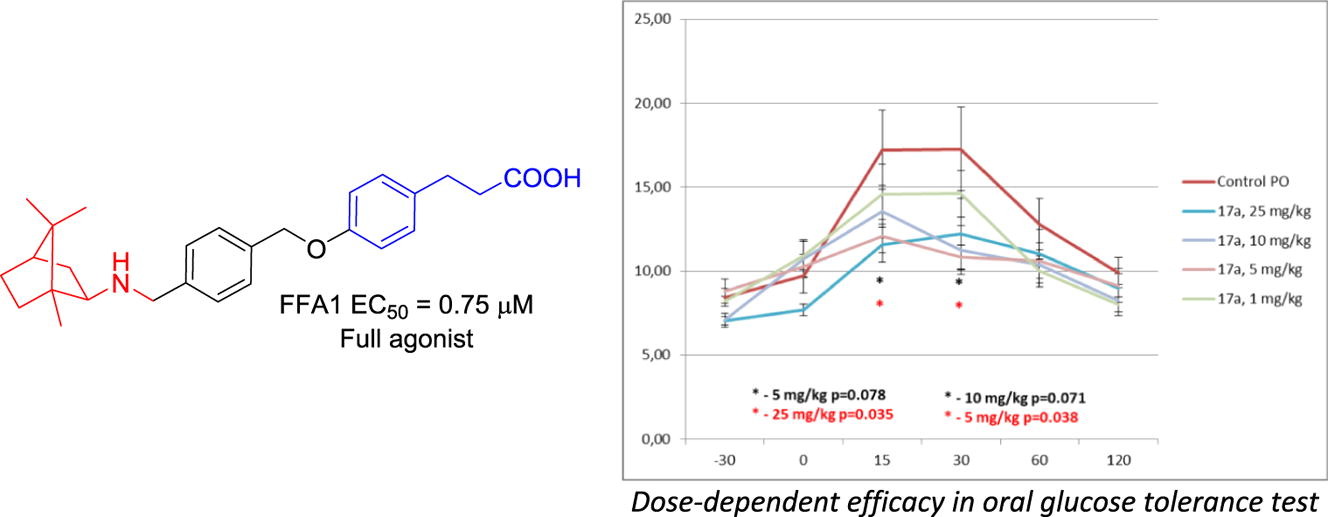
Abstract
Six derivatives of 3-phenylpropionic acid bearing various natural and natural-like, spatially defined peripheral motifs have been synthesized and evaluated in vitro for free fatty acid receptor 1 (FFA1) activation. Two frontrunner compounds (bearing a bornyl and cytosine groups) were evaluated in an oral glucose tolerance test in mice where both demonstrated the ability to sustain blood glucose levels following a glucose challenge. The bornyl compound displayed a somewhat superior, dose-dependent efficacy and, therefore, can be regarded as a lead compounds for further development as a therapeutic agent for type 2 diabetes mellitus. Its high affinity to FFA1 was rationalized by docking experiments.
Альметрики:








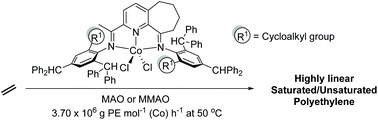
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}-9-{N(Ar)}C10H10N]CoCl2 (Ar = 2-(C5H9) -4,6-(CHPh2)2C6H2 Co1, 2-(C6H11)-4,6-(CHPh2)2C6H2 Co2, 2-(C8H15)-4,6-(CHPh2) 2C6H2 Co3, 2-(C12H23)-4,6-(CHPh2 )2C6H2 Co4, 2,6-(C5H9)2 -4-(CHPh2)C6H2 Co5). All five complexes have been characterized by a combination of FT-IR spectroscopy, elemental analysis and single crystal X-ray diffraction. The molecular structures of Co1, Co3 and Co5 highlight the substantial steric hindrance imparted by the 2-cycloalkyl-6-benzhydryl or 2,6-dicyclopentyl ortho-substitution pattern; distorted square pyramidal geometries are exhibited in each case. On activation with methylaluminoxane (MAO) or modified methylaluminoxane (MMAO), all the complexes (apart from Co4/MAO) were active ethylene polymerization catalysts (up to 3.70 ? 106 g PE per mol (Co) per h for Co5/MMAO), operating effectively at temperatures between 50 °C and 60 °C, producing polyethylenes with high molecular weights (up to 589.5 kg mol-1 for Co3/MAO). Furthermore, all polymers were highly linear (Tm > 130 °C) with narrow dispersities (Mw/Mn range: 2.0–3.0). The coexistence of two chain termination pathways, β-H elimination and transfer to aluminum, has been demonstrated using 13 C/1H NMR spectroscopy.
CMe}-9-{N(Ar)}C10H10N]CoCl2 (Ar = 2-(C5H9) -4,6-(CHPh2)2C6H2 Co1, 2-(C6H11)-4,6-(CHPh2)2C6H2 Co2, 2-(C8H15)-4,6-(CHPh2) 2C6H2 Co3, 2-(C12H23)-4,6-(CHPh2 )2C6H2 Co4, 2,6-(C5H9)2 -4-(CHPh2)C6H2 Co5). All five complexes have been characterized by a combination of FT-IR spectroscopy, elemental analysis and single crystal X-ray diffraction. The molecular structures of Co1, Co3 and Co5 highlight the substantial steric hindrance imparted by the 2-cycloalkyl-6-benzhydryl or 2,6-dicyclopentyl ortho-substitution pattern; distorted square pyramidal geometries are exhibited in each case. On activation with methylaluminoxane (MAO) or modified methylaluminoxane (MMAO), all the complexes (apart from Co4/MAO) were active ethylene polymerization catalysts (up to 3.70 ? 106 g PE per mol (Co) per h for Co5/MMAO), operating effectively at temperatures between 50 °C and 60 °C, producing polyethylenes with high molecular weights (up to 589.5 kg mol-1 for Co3/MAO). Furthermore, all polymers were highly linear (Tm > 130 °C) with narrow dispersities (Mw/Mn range: 2.0–3.0). The coexistence of two chain termination pathways, β-H elimination and transfer to aluminum, has been demonstrated using 13 C/1H NMR spectroscopy.