Up to 19/05/2022

Head - Cand. Sci.(Chem) Victor M. Tormyshev
Phone: (383) 330-49-81, internal phone: 3-00
e-mail:This email address is being protected from spambots. You need JavaScript enabled to view it.
The Group was founded in 1999.
Up to 01.06.2020 - Group of Metal Complex Catalysis (MCG)- Research areas:
Research areas:
Research areas:
- Spin probes for noninvasive diagnosis of oncologic diseases using the in vivo EPR Oxygen Imaging technologies.
- Spin probes for studying the biopolymer structure, reagents for studying the nuclear Overhauser effect.
- Synthesis of novel organic materials, including polyfunctional nitrogen-, oxygen- and sulfur-containing heterocyclic compounds (physiologically active compounds and synthons for drug production).
- Materials for microelectronics.
The most significant results
- Synthesis of various triarylmethyl (trityl, TAM) radicals, which are stable towards components of cytoplasm, perfectly soluble in water, and demonstrate ultra-narrow singlet signal in ESR spectra. Derivatives of trityls with a broad variation of hydrophilic properties. Synthesis of numerous hybrid trityl-nitroxide bi- and triradicals. These investigations are performed in collaboration with American and Israeli colleagues.
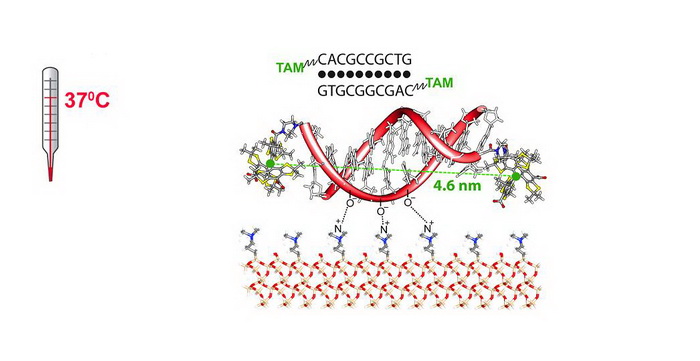
- An access to TAM-based spin- labels focused on SDSL of peptides and oligonucleotides. The first example of high-accuracy measurement of long – up to 46 Å - interspin distances in doubly labeled 10-mer DNAs at physiological temperature (37 oC) using SDSL and DQC ESR techniques. .
- Practical methods for synthesis of water-soluble polymeric electrolytes used in generation of ultra-thin defect-free nanolayers of transient metals.
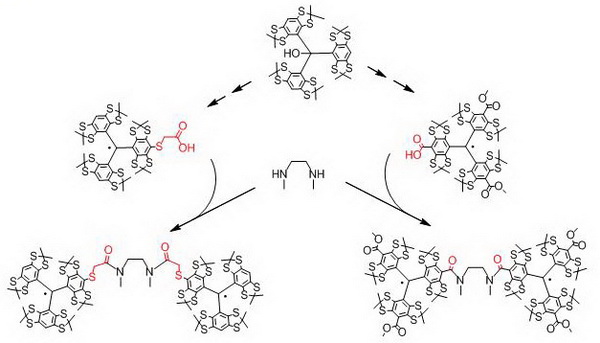
- Efficient approaches to synthesis of monofunctional derivatives of TAM and multi-spin systems
Project 13-04-00680 "Design of trityl spin labels with optimized properties and their application for structure elucidation of biomolecules using methods of dipole EPR spectroscopy"
The joint RFBR-NSF Project 14-03-93180 "International collaboration in chemistry: Dynamic nuclear polarization by nanoparticles and low-dimensionality aggregates"
Current Grants
- Grant P41 EB002034 from National Institute of Health “Center for Electron Paramagnetic Resonance Imaging in Vivo Physiology”, 2013-2018. The collaborative studies are performed by teams of the University of Chicago, the University of Denver, the University of Maryland, the University of Illinois at Urbana Shampaign (USA), and the Novosibirsk Institute of Organic Chemistry under the auspices of Center for EPR Imaging in vivo Physiology: http://epri.uchicago.edu/. The information resulted from these investigations is crucial for understanding of cancer physiology, radiological treatment improvement, study of stroke, heart attack, peripheral vascular disease and other biomedical problems.
- Project 13-04-00680 "Design of trityl spin labels with optimized properties and their application for structure elucidation of biomolecules using methods of dipole EPR spectroscopy" supported by the Russian Foundation for Basic Research, 2013-2015. The multi-disciplinary study is performed by teams of the Novosibirsk Institute of Organic Chemistry, the International Tomography Center and the Institute of Chemical Biology and Fundamental Medicine.
- The joint RFBR-NSF Project 14-03-93180 "International collaboration in chemistry: Dynamic nuclear polarization by nanoparticles and low-dimensionality aggregates", 2014-2016. The multi-disciplinary study is performed by teams of the Novosibirsk Institute of Organic Chemistry, the Institute of Chemical Kinetics and Combustion and the University of Alabama
- STAFF
Staff
№ Employee Position Room Phone Phone in. email Publications by years No users - PUBLICATIONS
Publications over the past years
Laboratory staff publications (DB NIOCh)
Reviews, Articles
2022- S. Ketter, M. Dajka, O. Rogozhnikova, S.A. Dobrynin, V.M. Tormyshev, E.G. Bagryanskaya, B. Joseph
In situ distance measurements in a membrane transporter using maleimide functionalized orthogonal spin labels and 5-pulse electron-electron double resonance spectroscopy
Journal of Magnetic Resonance Open (companion title to the Journal of Magnetic Resonance), Volumes 10-11, June 2022, 100041 doi:10.1016/j.jmro.2022.100041 - N.B. Asanbaeva, A.A. Sukhanov, A.A. Diveikina, O.Y. Rogozhnikova, D.V. Trukhin, V.M. Tormyshev, A.S. Chubarov, A.G. Maryasov, A.M. Genaev, A.V. Shernyukov, G.E. Salnikov, A.A. Lomzov, D.V. Pyshnyi, E.G. Bagryanskaya
Application of W-band 19F electron nuclear double resonance (ENDOR) spectroscopy to distance measurement using a trityl spin probe and a fluorine label
Phys. Chem. Chem. Phys., 2022, 24 (10), 5982-6001 doi:10.1039/D1CP05445G, IF=3.945 - I.O. Timofeev, L.V. Politanskaya, E.V. Tretyakov, Yu.F. Polienko, V.M. Tormyshev, E.G. Bagryanskaya, O.A. Krumkacheva, M.V. Fedin
Fullerene-based triplet spin labels: methodology aspects for pulsed dipolar EPR spectroscopy
Phys. Chem. Chem. Phys., 2022, V.24, N 7, Pp. 4475-4484 doi:10.1039/D1CP05545C, IF=3.945
2021- Trityl radicals: synthesis, properties, and applications/ V. M. Tormyshev & E. G. Bagryanskaya// Russian Chemical Bulletin, 2021, V. 70, N 12, Pp 2278-2297 doi:10.1007/s11172-021-3345-6, IF=1.222
- A Simple and Convenient Synthesis of a Multifunctional Spin Probe, Phosphonate Derivative of a Persistent Radical of the Triarylmethyl Series/ O. Yu. Rogozhnikova, D. V. Trukhin, N. B. Asanbaeva, V. M. Tormyshev// Russian Journal of Organic Chemistry, 2021, V. 57, Pp 905-913 doi:10.1134/S107042802106004X, IF=0.723
- B. Joseph, S. Ketter, A. Gopinath, O. Rogozhnikova, D. Trukhin, V.M. Tormyshev, E.G. Bagryanskaya
In situ labeling and distance measurements of membrane proteins in E coli using Finland and OX063 trityl labels
Chemistry - A European Journal, 2021, V. 27, N 7, Pp 2299-2304 doi:10.1002/chem.202004606, IF=4.857 - A. Chubarov, A. Spitsyna, O. Krumkacheva, D. Mitin, D. Suvorov, V. Tormyshev, M. Fedin, M.K. Bowman, E. Bagryanskaya
Reversible Dimerization of Human Serum Albumin
Molecules 2021, 26(1), 108 doi:10.3390/molecules26010108, IF=4.411
2020- Novel Acetylene Derivatives of Stable Tetrathiatriarylmethyl Radicals/ D. V. Trukhin, O. Yu. Rogozhnikova, T. I. Troitskaya, S. S. Ovcherenko, E. V. Amosov, V. M. Tormyshev// Russian Journal of Organic Chemistry, 2020, V. 56, N 11, Pp 1905-1910 doi:10.1134/S1070428020110032, IF=0.624
- M. Bretschneider, P. E Spindler, O.Yu. Rogozhnikova, D.V. Trukhin, B. Endeward, A.A. Kuzhelev, E.G. Bagryanskaya, V.M. Tormyshev, T.F. Prisner
Multi-Quantum Counting of Trityl Radicals
The Journal of Physical Chemistry Letters, 2020, 11, 15, 6286-6290 doi:10.1021/acs.jpclett.0c01615, IF=6.71 - V. Tormyshev, A. Chubarov, O. Krumkacheva, D. Trukhin, O. Rogozhnikova, A. Spitsyna, A. Kuzhelev, V. Koval, M. Fedin, T. Godovikova, M. Bowman, E.G. Bagryanskaya
Methanethiosulfonate Derivative of OX063 Trityl: a Promising and Efficient Reagent for SDSL of Proteins
Chemistry-A European Journal, 2020, V.26, N 12, Pp 2705-2712 doi:10.1002/chem.201904587, IF=4.857 - A.A. Kuzhelev, V.M. Tormyshev, V.F. Plyusnin, O.Yu. Rogozhnikova, M.V. Edeleva, S.L. Veber, E.G. Bagryanskaya
Photochemistry of tris(2,3,5,6-tetrathiaaryl)methyl radicals in various solutions
Phys. Chem. Chem. Phys., 2020, V. 22, N 3, Pp 1019-1026 doi:10.1039/C9CP06213K, IF=3.43
2019- O.A. Krumkacheva, G.Yu. Shevelev, A.A. Lomzov, N.S. Dyrkheeva, A.A. Kuzhelev, V.V. Koval, V.M. Tormyshev, Yu.F. Polienko, M.V. Fedin, D.V. Pyshnyi, O.I. Lavrik, E.G. Bagryanskaya
DNA complexes with human apurinic/apyrimidinic endonuclease 1: structural insights revealed by pulsed dipolar EPR with orthogonal spin labeling
Nucleic Acids Research,2019, V. 47, N 15, Pp 7767-7780 doi:10.1093/nar/gkz620, IF=11.147 - O.A. Krumkacheva, I.O. Timofeev, L.V. Politanskaya, Yu.F. Polienko, E.V. Tretyakov, O.Yu. Rogozhnikova, D.V. Trukhin, V.M. Tormyshev, A.S. Chubarov, E.G. Bagryanskaya, M.V. Fedin
Triplet Fullerenes as Prospective Spin Labels for Nanoscale Distance Measurements by Pulsed Dipolar EPR
Angewandte Chemie International Edition, 2019, V. 58, N 38, Pp 13271-13275 doi:10.1002/anie.201904152, IF=12.256 - K. Sato, R. Hirao, I. Timofeev, O.A. Krumkacheva, E. Zaytseva, O.Yu. Rogozhnikova, V.M. Tormyshev, D.V. Trukhin, E.G. Bagryanskaya, T. Gutmann, V. Klimavicius, G. Buntkowsky, K. Sugisaki, Sh. Nakazawa, H. Matsuoka, K. Toyota, D. Shiomi, T. Takui
Trityl-Aryl-Nitroxide Based Genuinely g-Engineered Biradicals, as Studied by Dynamic Nuclear Polarization, Multi-Frequency ESR/ENDOR, Arbitrary Wave Generator Pulse Microwave Waveform Spectroscopy and Quantum Chemical Calculations
The Journal of Physical Chemistry A, 2019, V. 123, N 34, Pp 7507-7517 doi:10.1021/acs.jpca.9b07169, IF=2.641 - New Spin Probes: Triand Hexacationic Derivatives of Persistent Tris(tetrathioaryl)methyl Radicals/ D.V. Trukhin, O.Yu.Rogozhnikova, T.I. Troitskaya, A.A, Kuzhelev, E.V. Amosov,H.J. Halpernc, V.V. Koval'b, V.M. Tormyshev// Russian Journal of Organic Chemistry, 2019, V. 55, N 3, pp 296-301 doi:10.1134/S1070428019030035, IF=0.751
- L. Lampp, O.Yu. Rogozhnikova, D.V. Trukhin, V.M. Tormyshev, M.l K. Bowman, N. Devasahayam, M.C. Krishna, K. Mader, P. Imming
A radical containing injectable in-situ-oleogel and emulgel for prolonged in-vivo oxygen measurements with CW EPR
Free Radical Biology and Medicine, 2019, V. 130, Pp 120-127 doi:10.1016/j.freeradbiomed.2018.10.442, IF=5.657
2018- M.V. Edeleva, S.R-A. Marque, O.Yu. Rogozhnikova, V.M. Tormyshev, T.I. Troitskaya, E.G. Bagryanskaya
Radical polymerization of radical‐labeled monomers: The triarylmethyl‐based radical monomer as an example
Journal of Polymer Science Part A: Polymer Chemistry, 2018, V. 56, N 23, Pp 2656-2664 doi:10.1002/pola.29249, IF=2.588 - A.A. Kuzhelev, O.A. Krumkacheva, I.O. Timofeev, V.M. Tormyshev, M.V. Fedin, E.G. Bagryanskaya
Electron-Spin Relaxation of Triarylmethyl Radicals in Glassy Trehalose
Applied Magnetic Resonance, 2018, V. 49, N 11, pp 1171-1180 doi:10.1007/s00723-018-1023-0, IF=0.835 - A.A. Kuzhelev, O.A. Krumkacheva, M.Yu. Ivanov, S.A. Prikhod'ko, N. Yu. Adonin, V.M. Tormyshev, M. K Bowman, M.V. Fedin, E.G. Bagryanskaya
Pulse EPR of Triarylmethyl Probes: New Approach for Investigation of Molecular Motions in Soft Matter
J. Phys. Chem. B, 2018, 122 (36), pp 8624–8630 doi:10.1021/acs.jpcb.8b07714, IF=3.145 - S. Bothe, J. Nowag, V. Klimavicius, M. Hoffmann, T.I. Troitskaya, E.V. Amosov, V.M. Tormyshev, I. Kirilyuk, A. Taratayko, A.A. Kuzhelev, D. Parkhomenko, E.G. Bagryanskaya, T. Gutmann, G. Buntkowsky
Novel Biradicals for Direct Excitation Highfield Dynamic Nuclear Polarization
J. Phys. Chem. C, 2018, 122 (21), pp 11422-11432 doi:10.1021/acs.jpcc.8b02570, IF=4.484 - G.Yu. Shevelev, E.L. Gulyak, A.A. Lomzov, A.A. Kuzhelev, O.A. Krumkacheva, M.S. Kupryushkin, V.M. Tormyshev, M.V. Fedin, E.G. Bagryanskaya, D. V. Pyshnyi
A Versatile Approach to Attachment of Triarylmethyl Labels to DNA for Nanoscale Structural EPR Studies at Physiological Temperatures
J. Phys. Chem. B, 2018, 122 (1), pp 137-143 doi:10.1021/acs.jpcb.7b10689, IF=3.146
2017- B. Epel, M. Krzykawska-Serda, V. Tormyshev, M.C. Maggio, E.D. Barth, C.A. Pelizzari, H.J. Halpern
Spin Lattice Relaxation EPR pO2 Images May Direct the Location of Radiation Tumor Boosts to Enhance Tumor Cure
, 2017, V. 75, N 3-4, pp 295-298 doi:10.1007/s12013-017-0825-2, IF=1.32 - A.A. Kuzhelev, V.M. Tormyshev, O.Yu. Rogozhnikova, D.V. Trukhin, T.I. Troitskaya, R.K. Strizhakov, O.A. Krumkacheva, M.V. Fedin, E.G. Bagryanskaya
Triarylmethyl Radicals: An EPR Study of 13C Hyperfine Coupling Constants
Zeitschrift für Physikalische Chemie, 2017, V. 231, N 4, Pp.777-794 doi:10.1515/zpch-2016-0811, IF=1.327
2016- G. Audran, E.G. Bagryanskaya, P. Bremond, M.V. Edeleva, S.R.A. Marque, D.A. Parkhomenko, O.Yu. Rogozhnikova, V.M. Tormyshev, E.V. Tretyakov, D.V. Trukhin, S.I. Zhivetyeva
Trityl-based alkoxyamines as NMP controllers and spin-labels
Polym. Chem., 2016, 42(7), 6490-6499 doi:10.1039/C6PY01303A, IF=5.687 - M.V. Fedin, G. Yu. Shevelev, D. V. Pyshnyi, V.M. Tormyshev, G. Jeschke, M. Yulikov, E.G. Bagryanskaya
Interaction of Triarylmethyl Radical with DNA Termini Revealed by Orientation-Selective W-band Double Electron-Electron Resonance Spectroscopy
Phys. Chem. Chem. Phys., 2016,18(42), 29549-29554l doi:10.1039/C6CP05904J, IF=4.448 - B. Joseph, V.M. Tormyshev, O.Yu. Rogozhnikova, D. Akhmetzyanov, E.G. Bagryanskaya, T.F. Prisner
Selective High-Resolution Detection of Membrane Protein–Ligand Interaction in Native Membranes using Trityl-Nitroxide PELDOR
Angewandte Chemie International Edition, 2016, V, 55, N 38, Pp 11538-11542 doi:10.1002/anie.201606335, IF=11.709 - H. Chen, A.G. Maryasov, O. Rogozhnikova, D.V. Trukhin, V.M. Tormyshev, M. Bowman
Electron spin dynamics and spin-lattice relaxation of trityl radicals in frozen solutions
Phys. Chem. Chem. Phys., 2016,18, 24954-24965 doi:10.1039/C6CP02649D, IF=4.448 - Z. Yang, M.D. Bridges, C.J. Lopez, O.Yu. Rogozhnikova, D.V. Trukhin, E.K. Brooks, V. Tormyshev, H.J. Halpern, W.L. Hubbell
A Triarylmethyl Spin Label for Long-Range Distance Measurement at Physiological Temperatures Using T1 Relaxation Enhancement
Journal of Magnetic Resonance, V. 269, August 2016, Pp 50-54 doi:10.1016/j.jmr.2016.05.006, IF=2.888 - A.A. Kuzhelev, G. Yu. Shevelev, O.A. Krumkacheva, V.M. Tormyshev, D.V. Pyshnyi, M.V. Fedin, E.G. Bagryanskaya
Saccharides as Prospective Immobilizers of Nucleic Acids for Room-Temperature Structural EPR Studies
J. Phys. Chem. Lett., 2016, V. 7, N 12, pp 2544-2548 doi:10.1021/acs.jpclett.6b01024, IF=8.538 - Р.К. Стрижаков, А.А. Кужелев, В.М. Тормышев, Е.Г. Багрянская
Спектроскопия эпр тритильных радикалов: исследование особенностей сверхтонкого взаимодействия неспаренного электрона с ядрами 13С
Вестник Новосибирского государственного университета. Серия: Физика. 2016. Т. 11. № 1. С. 100-106. - D.V. Trukhin, O.Yu. Rogozhnikova, T.I. Troitskaya, V.G. Vasiliev, M.l K. Bowman, V.M. Tormyshev
Facile and High-Yielding Synthesis of TAM Biradicals and Monofunctional TAM Radicals
Synlett, 2016, 27(06), Pp 893-899 doi:10.1055/s-0035-1561299, IF=2.322 - B. Epel, G. Redler, V. Tormyshev, H.J. Halpern
Towards Human Oxygen Images with Electron Paramagnetic Resonance Imaging
Oxygen Transport to Tissue XXXVII, V.876 of the series Advances in Experimental Medicine and Biolog, Part VII, pp 363-369 doi:10.1007/978-1-4939-3023-4_45, IF=1.952 - B. Epel, G. Redler, C. Pelizzari, V.M. Tormyshev, H.J. Halpern
Approaching Oxygen-Guided Intensity-Modulated Radiation Therapy
Oxygen Transport to Tissue XXXVII, V.876 of the series Advances in Experimental Medicine and Biolog, Part IV, pp 185-193 doi:10.1007/978-1-4939-3023-4_23, IF=1.952
2015- G. Yu. Shevelev, O. A. Krumkacheva, A. A. Lomzov, A. A. Kuzhelev, D. V. Trukhin, O. Yu. Rogozhnikova, V. M. Tormyshev, D. V. Pyshnyi, M. V. Fedin, E.G. Bagryanskaya
Triarylmethyl Labels: Toward Improving the Accuracy of EPR Nanoscale Distance Measurements in DNAs
J. Phys. Chem. B, 2015, 119 (43), pp 13641-13648 doi:10.1021/acs.jpcb.5b03026, IF=3.302 - A.A. Kuzhelev, D.V. Trukhin, O.A. Krumkacheva, R.K. Strizhakov, O.Yu. Rogozhnikova, T.I. Troitskaya, M.V. Fedin, V.M. Tormyshev, E. G. Bagryanskaya
Room-Temperature Electron Spin Relaxation of Triarylmethyl Radicals at the X- and Q-Bands
J. Phys. Chem. B, 2015, 119 (43), pp 13630-13640 doi:10.1021/acs.jpcb.5b03027, IF=3.302
2014- G.Yu. Shevelev, O.A. Krumkacheva, A.A. Kuzhelev, A.A. Lomzov, O.Yu. Rogozhnikova, D.V. Trukhin, T.I. Troitskaya, V.M. Tormyshev, M.V. Fedin, D.V. Pyshnyi, E.G. Bagryanskaya
Physiological- Temperature Distance Measurement in Nucleic Acid using Triarylmethyl-Based Spin Labels and Pulsed Dipolar EPR Spectroscopy.
J. Am. Chem. Soc., 2014, 136 (28), pp 9874-9877. doi:10.1021/ja505122n, IF=11.444 - V. M. Tormyshev, O.Yu. Rogozhnikova, M. K. Bowman, D. V. Trukhin, T. I. Troitskaya, V. G. Vasiliev, L. A. Shundrin, H. J. Halpern
Preparation of Diversely Substituted Triarylmethyl Radicals by the Quenching of Tris(2,3,5,6-tetrathiaaryl)methyl Cations with C-, N-, P-, and S-Nucleophiles
European Journal of Organic Chemistry, 2014, V. 2014, N 2, pp 371-380. doi:10.1002/ejoc.201301161, IF=3.154
2013- S.N. Trukhan, V.F. Yudanov, V.M. Tormyshev, O.Yu. Rogozhnikova, D.V. Trukhin, M.K. Bowman, M.D. Krzyaniak, H. Chen, O.N. Martyanov
Hyperfine interactions of narrow-line trityl radical with solvent molecules
Journal of Magnetic Resonance, 2013, V. 233, P. 29-36. doi:10.1016/j.jmr.2013.04.017, IF=2.299 - O.Yu. Rogozhnikova, V.G. Vasiliev, T.I. Troitskaya, D.V. Trukhin, T.V. Mikhalina, H.J. Halpern, V.M. Tormyshev
Generation of Trityl Radicals by Nucleophilic Quenching of Tris(2,3,5,6-tetrathiaaryl)methyl Cations and Practical and Convenient Large-Scale Synthesis of Persistent Tris(4-carboxy-2,3,5,6-tetrathiaaryl)methyl Radical
European Journal of Organic Chemistry, 2013, V. 2013, N 16, P. 3347-3355. doi:10.1002/ejoc.201300176, IF=3.344
2012- V.M. Tormyshev, A.M. Genaev, G.E. Sal'nikov, O.Yu. Rogozhnikova, T.I. Troitskaya, D.V. Trukhin, V.I. Mamatyuk, D.S. Fadeev, H.J. Halpern
Triarylmethanols with Bulky Aryl Groups and the NOESY/EXSY Experimental Observation of a Two-Ring-Flip Mechanism for the Helicity Reversal of Molecular Propellers
Eur.J. Org. Chem., 2012, N 3, 623-629. doi:10.1002/ejoc.201101243, IF=3.206
2010- R. Halevy, V. Tormyshev, A. Blank
Microimaging of Oxygen Concentration near Live Photosynthetic Cells by Electron Spin Resonance
Biophys. J., 2010, V. 99, N 3, 971–978. doi:10.1016/j.bpj.2010.05.002, IF=4.389 - Y. Talmon, L. Shtirberg, W. Harneit, O.Yu. Rogozhnikova, V. Tormyshev, A. Blank
Molecular diffusion in porous media by PGSE ESR
Chem.Phys.Phys.Chem., 2010, 12(23), 5998–6007. doi:10.1039/b922060g, IF=4.116
2006- V.M. Tormyshev, D.V. Trukhin, O.Yu. Rogozhnikova, T.V. Mikhalina, T.I. Troitskaya, A. Flinn
Aryl Alkyl Ketones in One-Pot Gewald Synthesis of 2-Aminothiophenes
SynLett., 2006, V. 3, N 16, 2559-2564. doi:10.1055/s-2006-951484, IF=2.69 - A combinatorially convenient version of synthesis of 5-substituted oxazole-4-carboxylic acid ethyl esters/ V. M. Tormyshev, T. V. Mikhalina, O. Yu. Rogozhnikova, T. I. Troitskaya, D. V. Trukhin// Russian Journal of Organic Chemistry, 2006, V. 42, N 7, pp 1031-1035 doi:10.1134/S1070428006070177, IF=0.419
- S. Ketter, M. Dajka, O. Rogozhnikova, S.A. Dobrynin, V.M. Tormyshev, E.G. Bagryanskaya, B. Joseph
Joomla Plugins


