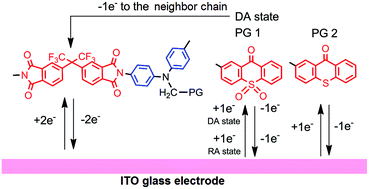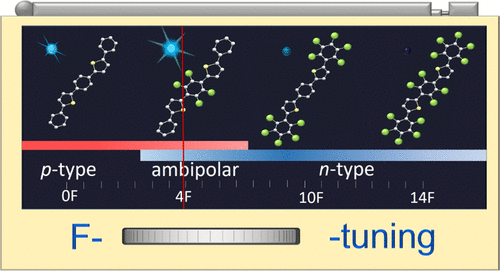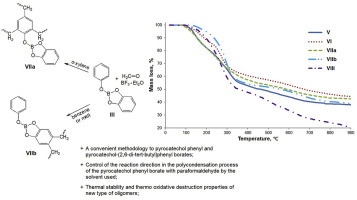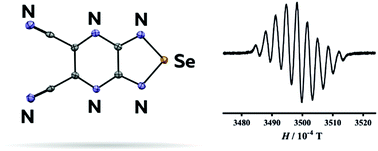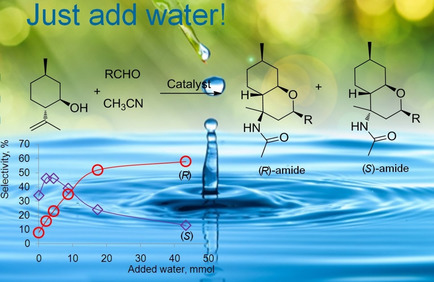
Just add water! A range of heterogeneous ‐SO3H functionalized catalysts were investigated for the first time using the Prins‐Ritter (−)‐isopulegol reaction with benzaldehyde and acetonitrile as a model producing 4‐amido derivatives of octahydro‐2H‐chromenes (as S‐ and R‐diastereomers). A strong effect of water addition prior the reaction on the overall selectivity and the ratio of isomers was found.
На сайте журнала ChemCatChem (IF 4,495) опубликована статья с участием сотрудников Института: Н.С. Ли-Жуланова (мнс, ЛНТПС), д.х.н., проф. РАН К.П. Волчо (гнс, ЛФАФ) и д.х.н., член.-корр. РАН, поф. Н.Ф. Салахутдинова (зав .отделом медицинской химии)
Stereoselectivity Inversion by Water Addition in the −SO3H‐catalyzed Tandem Prins‐Ritter Reaction for Synthesis of 4‐amidotetrahydropyran Derivatives
Dr. Alexander Yu. Sidorenko, Nikolai S. Li‐Zhulanov, Päivi Mäki‐Arvela, Thomas Sandberg, Anna V. Kravtsova, Andreia F. Peixoto, Cristina Freire, Konstantin P. Volcho, Nariman F. Salakhutdinov, Vladimir E. Agabekov, Prof. Dmitry Yu. Murzin
ChemCatChem, Volume12, Issue 9, May 7, 2020, Pages 2605-2609
First published: 05 Februaryr 2019
doi: 10.1002/cctc.202000070
Abstract
A range of heterogeneous ‐SO3H functionalized catalysts including carbon and halloysite nanotubes, commercial K10 clay, Amberlyst‐15 etc. was investigated for the first time using as a model the Prins‐Ritter reaction of (−)‐isopulegol with benzaldehyde and acetonitrile producing 4‐amido derivatives of octahydro‐2H‐chromenes (as (S)‐ and (R)‐diastereomers). A strong effect of water addition prior the reaction on the overall selectivity and the ratio of isomers in the case of heterogeneous and homogeneous (p‐toluenesulfonic acid) catalysis was found for the first time. The yield of the (R)‐diastereomer sharply increased with increasing amount of added water, while the S‐isomer prevailed with a minimum amount of added water. Experimental results and DFT calculations clearly indicate a kinetic control for R‐amide formation. Typically synthesis of 4‐amidooctahydro‐2H‐chromenes requires subzero temperatures and toxic catalysts, which were avoided in the current work. Nevertheless, the yield of the desired products (up to 83 %) at 30 °C after water addition exceeded the values reported previously. Thus, adding water is a simple and a very effective method for controlling both the yield and stereoselectivity of the Prins‐Ritter reaction products under mild conditions.
Альметрики:









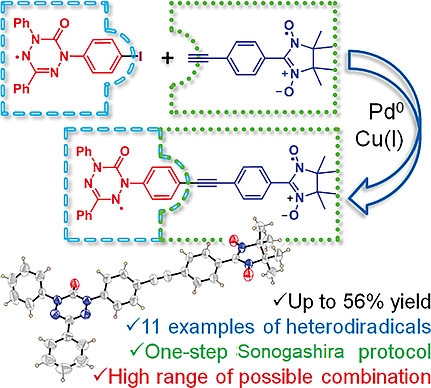



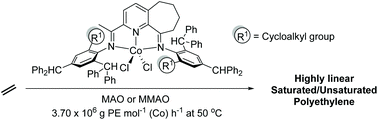
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CMe}-9-{N(Ar)}C10H10N]CoCl2 (Ar = 2-(C5H9) -4,6-(CHPh2)2C6H2 Co1, 2-(C6H11)-4,6-(CHPh2)2C6H2 Co2, 2-(C8H15)-4,6-(CHPh2) 2C6H2 Co3, 2-(C12H23)-4,6-(CHPh2 )2C6H2 Co4, 2,6-(C5H9)2 -4-(CHPh2)C6H2 Co5). All five complexes have been characterized by a combination of FT-IR spectroscopy, elemental analysis and single crystal X-ray diffraction. The molecular structures of Co1, Co3 and Co5 highlight the substantial steric hindrance imparted by the 2-cycloalkyl-6-benzhydryl or 2,6-dicyclopentyl ortho-substitution pattern; distorted square pyramidal geometries are exhibited in each case. On activation with methylaluminoxane (MAO) or modified methylaluminoxane (MMAO), all the complexes (apart from Co4/MAO) were active ethylene polymerization catalysts (up to 3.70 ? 106 g PE per mol (Co) per h for Co5/MMAO), operating effectively at temperatures between 50 °C and 60 °C, producing polyethylenes with high molecular weights (up to 589.5 kg mol-1 for Co3/MAO). Furthermore, all polymers were highly linear (Tm > 130 °C) with narrow dispersities (Mw/Mn range: 2.0–3.0). The coexistence of two chain termination pathways, β-H elimination and transfer to aluminum, has been demonstrated using 13 C/1H NMR spectroscopy.
CMe}-9-{N(Ar)}C10H10N]CoCl2 (Ar = 2-(C5H9) -4,6-(CHPh2)2C6H2 Co1, 2-(C6H11)-4,6-(CHPh2)2C6H2 Co2, 2-(C8H15)-4,6-(CHPh2) 2C6H2 Co3, 2-(C12H23)-4,6-(CHPh2 )2C6H2 Co4, 2,6-(C5H9)2 -4-(CHPh2)C6H2 Co5). All five complexes have been characterized by a combination of FT-IR spectroscopy, elemental analysis and single crystal X-ray diffraction. The molecular structures of Co1, Co3 and Co5 highlight the substantial steric hindrance imparted by the 2-cycloalkyl-6-benzhydryl or 2,6-dicyclopentyl ortho-substitution pattern; distorted square pyramidal geometries are exhibited in each case. On activation with methylaluminoxane (MAO) or modified methylaluminoxane (MMAO), all the complexes (apart from Co4/MAO) were active ethylene polymerization catalysts (up to 3.70 ? 106 g PE per mol (Co) per h for Co5/MMAO), operating effectively at temperatures between 50 °C and 60 °C, producing polyethylenes with high molecular weights (up to 589.5 kg mol-1 for Co3/MAO). Furthermore, all polymers were highly linear (Tm > 130 °C) with narrow dispersities (Mw/Mn range: 2.0–3.0). The coexistence of two chain termination pathways, β-H elimination and transfer to aluminum, has been demonstrated using 13 C/1H NMR spectroscopy.